December 2, 2024 Public Hearing on Proposal to Reschedule Marijuana
WFQA, LLC Regulatory Compliance Officer
On August 29, 2024 the Drug Enforcement Administration (DEA) announced that a hearing on the rescheduling of marijuana will be held on December 2, 2024, at its headquarters in Arlington, VA. The length of the hearing, which is intended to gather input from parties who would be negatively affected by the proposed rescheduling, is at the discretion of the hearing official and could take one or multiple days. The DEA administrator will choose who can speak at the hearing. The deadline to file a "notice of appearance" was September 30. More details on the notice of the hearing can be found at: https://www.federalregister.gov/documents/2024/08/29/2024-19370/schedules-of-controlled-substances-rescheduling-of-marijuana
The rescheduling of marijuana was initially proposed in a Notice of Proposed Rulemaking (NPRM) published in the Federal Register on May 21, 2024. The public comment period on the NPRM closed July 22, 2024. There were over 22,000 comments submitted to the NPRM docket.
Early news reports, largely fueled by the cannabis industry lobby, suggested this change was imminent and would probably happen before the presidential election in November 2024. The DEA's granting of a hearing date in December will likely postpone any DEA decisions on the rulemaking until sometime in 2025.
MRO Responsibilities Under
10 CFR 26 (NRC) Fitness for Duty Regulation:
Medication Misuse
The March 2024 MROCC MRO Quarterly 2024 newsletter included a detailed discussion of the MRO role in determining subversion vs. substitution and adulteration under Nuclear Regulatory Commission (NRC) regulations (10 CFR 26). Differences in MRO interpretation of specimens was compared to other Federal workplace drug testing programs (DOT, HHS). Another major difference between 10 CFR 26 and the other Federally regulated workplace drug testing programs is the option for the MRO to decide that the donor's use of a legal medication is "misuse" rather than "negative" or "positive." Following a determination of "misuse", the MRO can recommend follow-up testing to assure that no future "misuse" occurs. Generally, there are no adverse employment actions following the initial determination of "misuse".
10 CFR 26 is unique among the Federally regulated workplace drug testing programs in that "misuse" is specifically included in the regulation and licensees (employers required to have an operating license granted by the NRC) must include "misuse" in their written policies and procedures. Regarding "misuse", NRC regulations state the following:
§ 26.27 Written policy and procedures
§ 26.75 Sanctions
§ 26.185 Determining a fitness-for-duty policy violation
In addition to NRC regulations, the National Institute on Drug Abuse, Misuse of Prescription Drugs Research Report, June 2020 (https://nida.nih.gov/publications/research-reports/misuse-prescription-drugs/overview), states:
"Misuse of prescription drugs means taking a medication in a manner or dose other than prescribed; taking someone else's prescription, even if for https://nida.nih.gov/publications/research-reports/misuse-prescription-drugs/overview a legitimate medical complaint such as pain; or taking a medication to feel euphoria (i.e., to get high). The term nonmedical use of prescription drugs also refers to these categories of misuse.
The three classes of medication most commonly misused are:
- opioids - usually prescribed to treat pain
- central nervous system [CNS] depressants (this category includes tranquilizers, sedatives, and hypnotics) - used to treat anxiety and sleep disorders
- stimulants - most often prescribed to treat attention-deficit hyperactivity disorder (ADHD)"
Case Studies
Now some case studies to see how the MRO may interpret and implement 10 CFR 26 Fitness for Duty regulations regarding "misuse."
Prescription Medication Misuse: Amphetamines
CASE #1
Donor tests positive for amphetamine, has prescription for amphetamine ER 20 mg to be taken once daily.
However, during the MRO interview and verification of the prescription, it is noted that 30 pills have lasted > 9 months.
Donor readily admits that he uses this medication as a study aid only, to focus on test taking. Denies having diagnosis of ADD/ADHD or other health condition for which stimulants are recommended. Has not seen his provider since obtaining the prescription, unable to obtain letter from provider within 5 business days.
DISCUSSION:
The DEA lists amphetamines as a Schedule II substance, with a high potential for abuse, with use potentially leading to severe psychological or physical dependence.
The FDA has not authorized use of amphetamines as a "study aid" or "testing taking aid"
Such "Off Label" use is not recommended by clinical guidelines or professional standards of care.
Amphetamines have a significant potential for abuse and should only be taken under close medical supervision.
NOTE:
MRO declared this a "misuse" of prescription medication; f/u testing recommended and donor was informed that future misuse would be declared a violation. Donor stated he would stop medication. No further misuse has been identified.
CASE #2
Donor tests positive for amphetamine, has prescription for Vyvanse 50 mg to be taken once daily.
However, during the MRO interview and verification of the prescription, it is noted that a 90 day supply has lasted more than 5 months.
Donor was noted having been diagnosed with ADD/ADHD at age 18 and symptoms well controlled with medications (Adderall or Vyvanse) since that time. He was adamant that his prescribing physician has told him that he should take the medication "only when he feels like he needs it."
DISCUSSION:
ADD/ADHD is a chronic condition that impacts a person every day to some degree.
Neither the FDA nor any professional organization has recommended patients determine their own "as needed" medication schedule for treatment of ADD/ADHD.
Donor instructed to obtain letter from prescribing provider for MRO review within 5 business days.
MRO received letter from pediatrician (donor 24 years old, just completed college) stating donor had been authorized to take medication when he "feels like he needs it."
Telephone call with pediatrician for further clarification of diagnosis and treatment unproductive. Pediatrician told MRO: "You just don't understand how college students use amphetamines." MRO noted that pediatrician was not familiar with nuclear work environment and requirements of NRC regulations.
MRO referred donor for a mandatory EAP evaluation, to include medication review by psychiatrist.
Psychiatrist confirmed diagnosis of ADD and recommended DAILY use of Vyvanse.
NOTE:
MRO declared this a "misuse" of prescription medication; f/u testing recommended and donor informed that further misuse would be declared a violation. Donor required to be compliant with medication use every day that he was working; ok if he chose to not take medication on his days off. No further misuse has been identified.
Prescription Medication Misuse: Opioids
CASE #1
Donor tests positive for codeine, has prescription for promethazine with codeine, however, it is not in his name (son's cough medication).
DISCUSSION:
Always follow the regulation:
§ 26.185 Determining a fitness-for-duty policy violation
Most pharmacies place a comment on each dispensed container of a controlled substance, similar to: "Federal law prohibits the transfer of this drug to any person other than the patient for whom it is prescribed." As there was no clinical evidence of abuse, 10 CFR 26 mandates that this is "misuse."
NOTE:
MRO declared this a "misuse" of prescription medication; f/u testing recommended and donor was informed that further misuse would be declared a violation. No further misuse has been identified.
CASE #2
Donor tests positive for codeine, has prescription for Tylenol #3 to be taken 1-2 tablets every 4-6 hrs as needed for pain.
However, during the MRO interview and verification of the prescription, it is noted that this prescription had been filled 6 months earlier for an acute knee injury that had resolved in 4-6 weeks.
Donor initiated treatment of a recent ankle injury with left over medication without consulting the prescribing provider.
DISCUSSION:
Again, always follow the regulation:
§ 26.185 Determining a fitness-for-duty policy violation
The "intended medical purpose" of this medication was to treat an acute knee injury, not an ankle injury.
Only licensed health care providers WITH Federal and/or State DEA Registration may prescribe controlled substances.
Each state has regulations in place that forbid licensed health care providers with authority to prescribe controlled substances to self-prescribe controlled substances and to diagnose/treat family members for anything other than a minor illness. Certainly non-clinicians are not authorized to
self-prescribe controlled substances.
Donor was NOT currently under the care of the prescribing provider
The Opioid Epidemic continues unabated in the US, with > 100,000 overdose deaths annually. Careless use of opioid medications contributes to this epidemic.
NOTE:
MRO declared this a "misuse" of prescription medication; f/u testing recommended and donor was instructed that he needed to always consult his prescribing provider prior to initiating treatment with "left-over" medications. In addition, he was informed that further misuse would be declared a violation. No further misuse has been identified.
Many state Medical Boards have written policies regarding prescribing of opioid medications and providers have begun writing prescriptions highly specific in directing how medication is to be used, i.e. "oxycodone HCL 5 mg...Take one tablet by mouth every four hours for post-op pain for up to seven days." Any other use of the medication would be deemed "misuse," not consistent with "intended medical purpose" as outlined by the prescribing provider. Companies may wish to consider an educational program to alert workers to the issue of medication misuse.
Take Your Federal Blinders Off: Consider State Laws
"A person who is a licensed physician and who is responsible for receiving and reviewing laboratory results generated by an employer's drug testing program and evaluating medical explanations for certain drug test results."
Definition of Medical Review Officer found at 49 CFR part 40.3
Introduction
As I write this article it is nearly October 2024. Workplace drug testing in this country has been occurring for nearly forty years1 and things are evolving fast. There are approximately fifty-million drug tests conducted each year in our workplaces.2 Only about six million of those tests are federally regulated.3 The rest are likely governed by state or local law or applicable drug testing protocols.
Federal rules do not apply to the vast majority of the workplace drug tests conducted in this country. Medical Review Officers (MROs) would be wise to take their federal blinders off and become aware of the state and local rules that apply to non-regulated tests.
Background
Since 1986 employment drug and alcohol testing has evolved primarily as a state issue, driven in part since 19964 by the increased legalization of cannabis. When in 1989 the United States Supreme Court authorized workplace drug testing for the first time5 there were only twelve state laws addressing workplace drug testing. There were at that time less than one-hundred court decisions on the subject. Today there are well over 30,000 court and agency decisions and more than 600 state laws that in some way affect workplace drug testing programs.
State laws: What Rules Apply?
Today there are thirty-one states that are considered "mandatory" states, meaning if an employer wishes to conduct drug testing the state rules must be followed. Those states include the following:
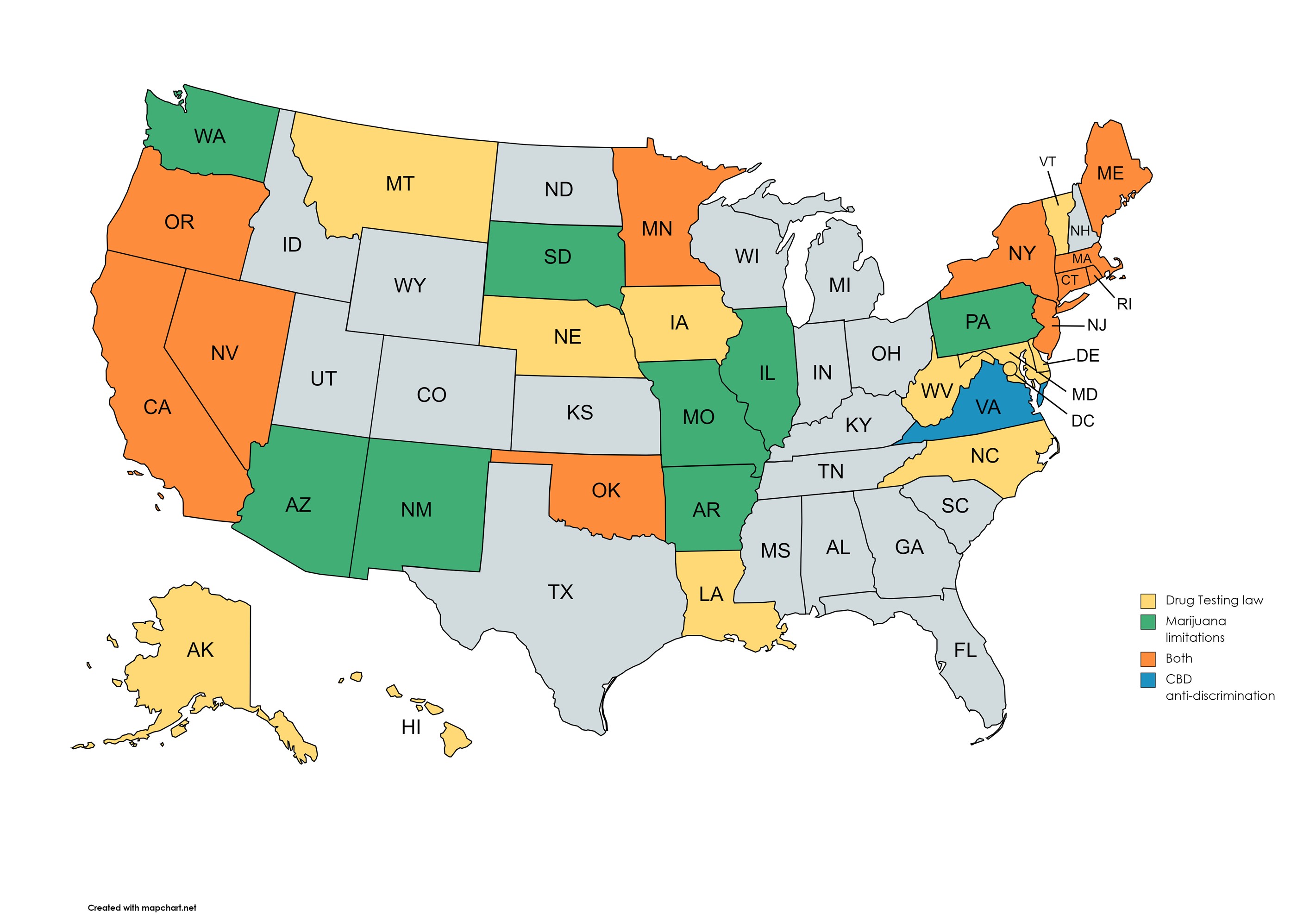
In nineteen of these states there is no requirement or reference to a medical review officer. But in over twenty-five state's laws or state's programs employers are required to use the services of an MRO. Here the rules may be different from those in the federal regulations. The state and local rules may differ as far as who can perform the services of an MRO, the requirements to become an MRO, and what may or may not be legal in the state.
Marijuana (Cannabis)6
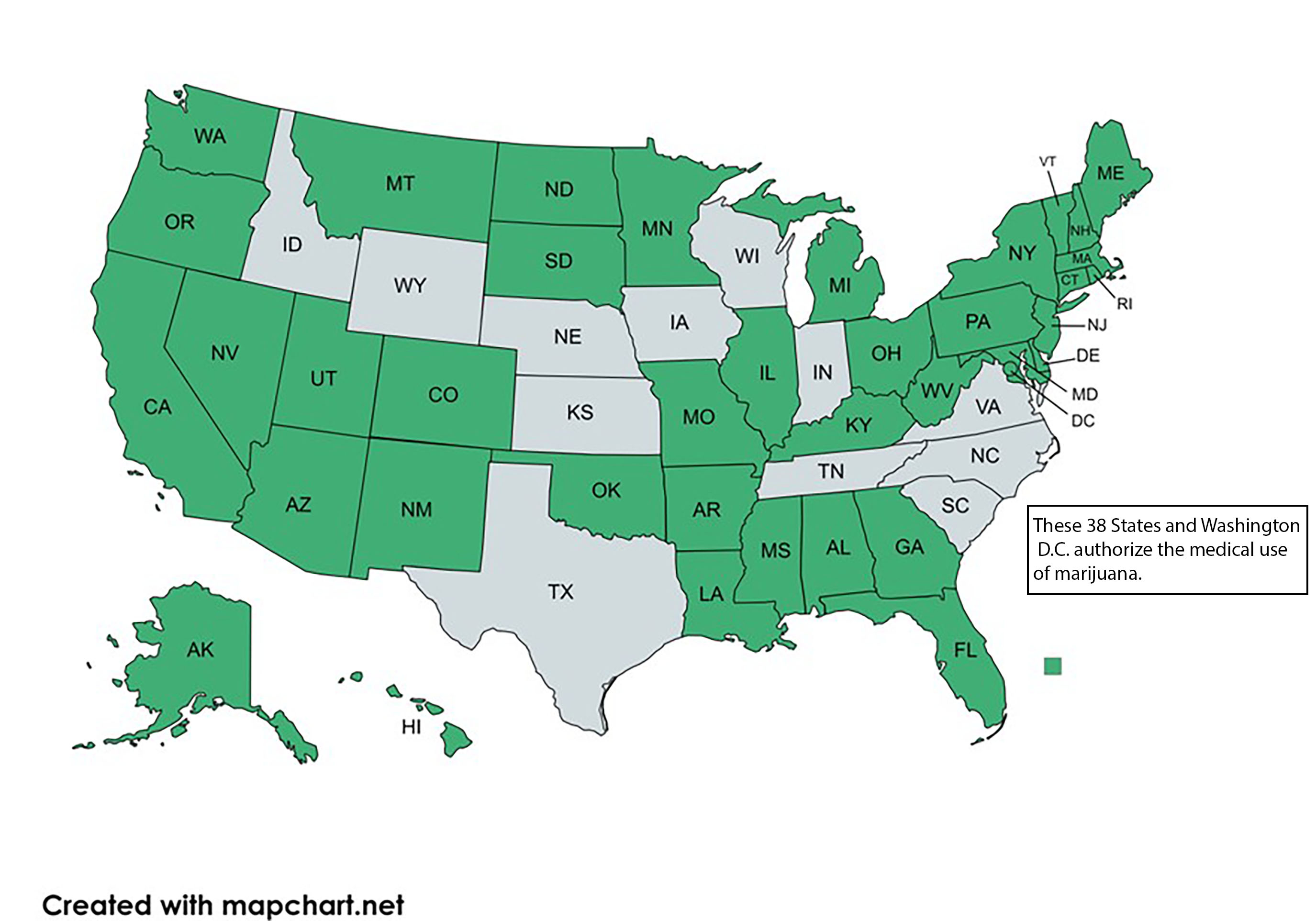
Perhaps the most impactful change on workplace drug testing over the past forty years is the ongoing legalization of marijuana. While marijuana remains an illegal drug under federal law, thirty-eight states have authorized its medical use.
Twenty-four states allow adult-use of cannabis.
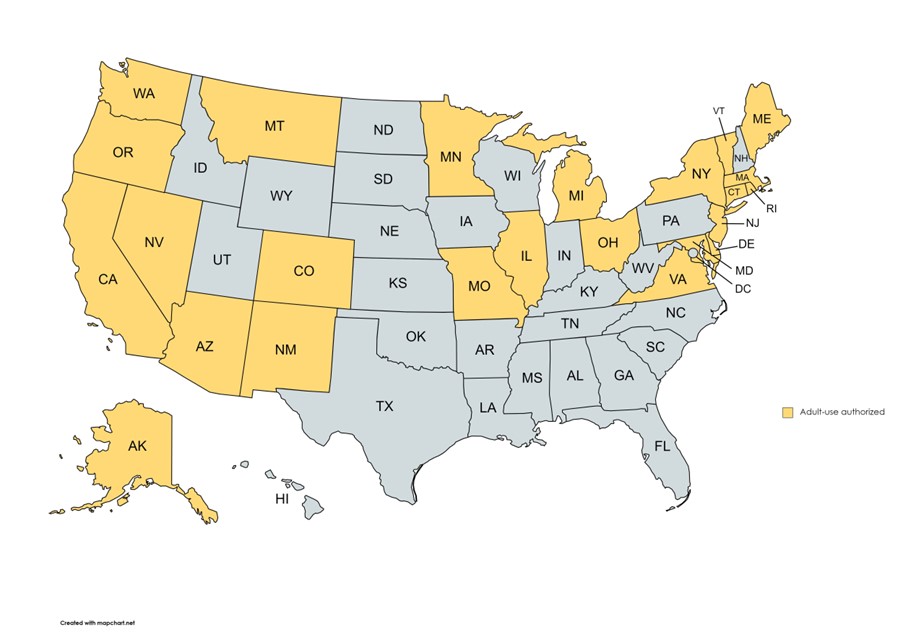
There are many differences between these state's laws. The details are beyond the scope of this article but what is critical to your role as MRO is recognizing that many of these state laws have rescheduled or decriminalized cannabis, at least for medical cannabis users.7
So, what does this mean for you as the MRO? For example, let's say you are reviewing a non-regulated test for an employer in South Dakota, the donor lives in South Dakota and is working in South Dakota and the donor is positive in the lab for cannabis.8 The donor presents you with a valid registry card authorizing her use of cannabis for medical purposes. What are you going to do?
South Dakota laws states as follows:
"SDCL 34-20G-22. Employment and drug testing. Except as provided in this chapter, a registered qualifying patient who uses cannabis for a medical purpose shall be afforded all the same rights under state and local law, as the person would be afforded if the person were solely prescribed a pharmaceutical medication, as it pertains to:
(1) Any interaction with a person's employer;
(2) Drug testing by a person's employer; or
(3) Drug testing required by any state or local law, agency, or government official."9
Are you obligated or expected to do anything different than you would under Part 40? Many MROs believe that this is the employer's problem. Do your employer customers believe that?
Consider the 2017 decision of the Supreme Judicial Court of Massachusetts10 in which they ruled for the employee and stated, "Under Massachusetts law... the use and possession of medically prescribed marijuana by a qualifying patient is as lawful as the use and possession of any other prescribed medication."
A Pennsylvania Court recently upheld an employee's unemployment claim when it found the MRO failed to follow the employer's policy with respect to a donor testing positive for marijuana.11 In that case the employer's policy prohibited marijuana use on or off the job. The company policy defined "illegal drugs" as any controlled substance (including the presence of their metabolites) of which the sale, possession or use is prohibited under state or federal law. But the company policy also provided as follows:
"[I]ndividuals may use legal over-the-counter medications or prescription drugs while at work strictly in accordance with the product instructions or a physician's prescription provided, however, that the use of such substances does not adversely affect the individual's ability to perform his or her job, or to do so in a safe manner. [citation excluded]. Further, the Drug Policy defines "legal drug" as "prescription medications and over-the-counter medications that have been legally obtained and are being used only in the manner, combination or quantity for which they were prescribed or manufactured." [citation excluded]. Moreover, Employer explained in its Drug Policy:
We recognize that employees may need to use legal drugs from time to time for medical reasons. The possession or use of legal drugs while on [Employer's] premises, during work hours and/or when performing any [Employer] business, including when driving vehicles, is permitted, provided such use or influence does not affect the safety of the employee, co-workers, customers or the public, an employee's job-performance or the safe or efficient operation of [Employer] facilities, equipment and vehicles.
The policy also indicated that any employee testing positive would be contacted by the company's MRO and provided an opportunity to the MRO "any medical reason that would account for" the positive result. The policy required the MRO to consider the individual's explanation and the MRO will determine if the explanation is acceptable.
The employee provided the MRO with a valid patient identification card to explain the use of the marijuana but the MRO forwarded the result as positive to the employer. The Court concluded...
"Consequently, because Claimant provided the MRO with his valid patient identification card to explain his use of prescribed medical marijuana, this Court cannot conclude that the MRO's reporting of Claimant's drug test as positive was "in accordance with employer's substance abuse policy." [citation omitted]. Accordingly, given the remedial nature of the Law to protect employees who become unemployed through no fault of their own,"[citation omitted] this Court holds that the UCBR did not err by granting Claimant UC benefits."
These are only a few examples that we have time for here but they are clearly not alone.
Under federal law because cannabis remains a Schedule I substance your obligation is clear. DOT's statement on medical marijuana includes the following:
"§40.151 What are MROs prohibited from doing as part of the verification process?
As an MRO, you are prohibited from doing the following as part of the verification process:
(e) You must not verify a test negative based on information that a physician recommended that the employee use a drug listed in Schedule I of the Controlled Substances Act. (e.g., under a state law that purports to authorize such recommendations, such as the "medical marijuana" laws that some states have adopted.)"12
Conclusion
The role of the medical review officer is vital to a successful workplace drug testing program. In fact I have said, "you don't have a drug test result until the MRO says you do."
But MROs must realize that the world of workplace drug testing is much larger than those tests conducted for federal purposes. Nearly eighty-seven percent of all workplace drug tests are not federally regulated. Federal rules don't apply.
Your review of each test must reflect the rules that apply to that test.
FOOTNOTES
- September 15, 1986, is the date of President Ronald Reagan's Executive Order 12,564 launching federal drug testing. That is generally considered the start of workplace drug testing in this country.
- https://www.slate.com/articles/health_and_science/cover_story/2015/12/workplace_drug_testing_is_widespread_but_ineffective.html, quoting Dr. Barry Sample who at the time was the Director of Science and Technology for the Employer Solutions business unit of Quest Diagnostics.
- See 87 Fed. Reg. 39039, (06/30/2022). Additionally there are an estimated 275,000 urine samples conducted for federal employees. See, 88 Fed. Reg. No. 196 at p 70768. According to the latest statistics there are more than 5 million commercial drivers registered with the FMCSA Clearinghouse. (https://clearinghouse.fmcsa.dot.gov/content/resources/Clearinghouse_MonthlyReport_July2024.pdf).
- On November 5, 1996,California became the first state to legalize cannabis use for medical purposes. See Ballot Proposition 215, now Health and Safety Code Sec. 11362.5 et seq.
- Skinner v. Ry. Labor Executives' Ass'n, 489 U.S. 602; 109 S. Ct. 1402; 103 L. Ed. 2d 639 (1989).
- As we learn more about the cannabis plant the trend is to refer to this drug as "cannabis." See https://www.nccih.nih.gov/health/cannabis-marijuana-and-cannabinoids-what-you-need-to-know#:~:text=The%20word%20%E2%80%9Ccannabis%E2%80%9D%20refers%20to,amounts%20of%20tetrahydrocannabinol%20(THC). Simply put, The word "cannabis" refers to all products derived from the plant Cannabis sativa. The word "marijuana" refers to parts of or products from the plant Cannabis sativa that contain substantial amounts of tetrahydrocannabinol (THC).
- For example see Alaska AS Sec. 11.71.090.
- A discussion of which metabolite (delta-9, hydroxy-THC or carboxy-THC) is beyond the scope of this article.
- SDCL 34-20G-23 provides, "The rights provided by §§ 34-20G-19 to 34-20G-25, inclusive, do not apply to the extent that they conflict with an employer's obligations under federal law or regulation or to the extent that they would disqualify an employer from a monetary or licensing-related benefit under federal law or regulation."
- Barbuto v. Advantage Sales & Mktg., LLC, 477 Mass. 456 (2017).
- The Pittsburgh Water and Sewer Authority, Petitioner, v. Unemployment Compensation Board of Review, 242 A.3d 704 (2020).
- https://www.transportation.gov/odapc/medical-marijuana-notice
DrugImpairment.com: Elevating Workplace Drug Impairment Training with Evidence-Based Solutions
As drug-related impairment continues to pose significant and evolving challenges to workplace safety, DrugImpairment.com is expanding its expertise into the occupational health space to deliver cutting-edge training programs and resources tailored specifically for employers and workers. Backed by years of experience in forensic toxicology, forensic drug recognition, and related sciences, the organization aims to address key gaps in current workplace drug impairment training with evidence-based, scientifically rigorous solutions.
A New Approach to Workplace Drug Impairment Training
Current options for workplace drug impairment training largely lack a strong, evidence-based curriculum and expert instruction from professionals with hands-on experience evaluating drug-impaired individuals. DrugImpairment.com seeks to address this gap by applying proven principles from clinical toxicology and forensic drug recognition - disciplines where evidence and accuracy are critical under the scrutiny of both scientific communities and the criminal justice system. Instructors that combine advanced scientific and clinical education with field law enforcement experience will provide a robust approach to drug impairment education.
DrugImpairment.com also recognizes the importance of addressing other types of impairment, such as fatigue, stress, and mental health issues. The organization aims to help supervisors understand these forms of impairment and distinguish the unique symptomatology patterns of drug impairment from other common causes. Training on intervention strategies and support for managing these additional impairments will also be provided.
Building on a Proven E-Learning Model
These new training offerings will build on the successful asynchronous e-learning model that has already proven highly effective for DrugImpairment.com's forensic sector clients. Recognizing that both industries face similar challenges with limited schedules and the need for convenient, accessible training, this flexible format is expected to be beneficial for gaining maximum reach in the occupational health space. By allowing participants to engage with the material at their own pace, this approach ensures broad accessibility without disrupting daily operations.
Collaboration with Medical Review Officers
As part of its development efforts, DrugImpairment.com recognizes the valuable role that Medical Review Officers (MROs) play. The organization sees MROs as key contributors who can help ensure that its programs align with the needs and challenges faced by employers across different industries. DrugImpairment.com is seeking MROs to collaborate in tailoring content for the diverse needs of different industries and adapt current forensic resources into valuable offerings for occupational health professionals.
The Future of Workplace Drug Impairment Training
DrugImpairment.com is committed to delivering high-quality, scientifically validated training that meets the needs of modern workplaces. By integrating expertise from forensic and clinical toxicology with law enforcement drug impairment recognition, along with insights from the occupational health sector, the organization aims to empower employers to identify and manage drug impairment effectively.
For MROs and others interested in contributing to these efforts, DrugImpairment.com welcomes their expertise and collaboration. Together, they can help shape the future of workplace safety by ensuring that the right training, tools, and resources are available to meet the challenges of today's workforce.
DrugImpairment.com's occupational health platform launches in 2025.
If you are interested in collaborating on this effort, you can:
- Take our MRO survey.
- Let us know you're interested in teaching.
- Join the waitlist for updates.
- Email Info@DrugImpairment.com with other questions or comments.
Hope Springs Eternal...
WFQA, LLC Regulatory Compliance Officer
As most of you know, in May 2023, the Department of Transportation (DOT) published a final rule that authorizes employers to use oral fluid drug testing as an alternative testing methodology to urine drug testing. This rulemaking incorporated many of the provisions of the HHS Oral Fluid Mandatory Guidelines (effective Jan 1, 2020) into 49 CFR Part 40. The Part 40 final rule had an effective date of June 2, 2023, but Oral Fluid (OF) testing cannot be conducted until at least 2 drug testing laboratories (one for primary specimen analysis, and another for split specimen reconfirmation testing) are HHS certified (approved) for oral fluid drug testing. DOT's Part 40 rule also placed additional requirements on the specimen collection devices that can be used for DOT testing, mainly that the oral fluid collected must be capable of being subdivided into two specimen vials during the collection, producing a primary and split specimen.
No laboratories are currently certified for oral fluid testing. Only one laboratory has even begun the application/approval process. Development, approval, manufacture and distribution of an oral fluid collection device (with buffer solution for stabilizing the collected oral fluid) that meets both FDA and DOT requirements has been very, very slow and cumbersome. The collection device must be FDA cleared, including clearance for the reagents to be used for OF initial testing at the laboratory.
And, finally there is the logistical challenge of getting oral fluid specimen collectors trained and qualified in accordance with the Part 40 requirements. Until there is an oral fluid split specimen collection device approved and available for widespread distribution, specimen collectors cannot complete the required training including the "mock collections".
On June 21, 2024 the DOT published some technical amendments to 49 CFR Part 40 and an NPRM that proposes: (a) temporary qualification requirements for mock oral fluid monitors, (b) privacy requirements by identifying which individuals may be present during an oral fluid collection, and (c) procedures for how collectors are to specify that a sufficient volume of oral fluid was collected. The DOT is expected to evaluate the comments to the NPRM and make decisions about the proposed changes to Part 40 in the coming months.
So, where does that leave us this October 2024? In the opinion of this long retired federal bureaucrat, I venture a guess that we will not see DOT oral fluid testing implemented in 2024. It is hoped by many that there may be approved split specimen collection devices, two certified laboratories, and specimen collectors qualified and trained by the end of 2nd Quarter 2005. As Alexander Pope said in his poem "An Essay on Man"... "Hope springs eternal in the human breast: Man never is, but always to be blest." This line represents the persistent human quality of optimism, the belief that situations can improve regardless of present difficulties or challenges.
ODAPC - DOT
- Office of Drug & Alcohol Policy & Compliance (ODAPC)
- DOT 49 CFR Part 40 Procedures for Transportation Workplace Drug and Alcohol Testing Programs
- Subscribe to the ODAPC Updates and News
- 2024 DOT Random Testing Rates
SAMHSA - HHS
- Medical Review Officer Guidance Manual for Federal Workplace Drug Testing Programs
- Mandatory Guidelines for Federal Workplace Drug Testing Programs using Urine (UrMG)
- Mandatory Guidelines for Federal Workplace Drug Testing Programs using Oral Fluid (OFMG)
NRC
CUSTODY AND CONTROL FORMS (CCFs)
- 2020 Federal CCF for Urine and Oral Fluid Specimens
- 2020 Guidance for Using the Federal CCF for Urine Specimens



Medical Review Officer
Certification Council (MROCC)
3231 S Halsted St, Ste Front #167
Chicago, IL 60608
Tel: 847.631.0599
Email: mrocc@mrocc.org
Co-editor: James Ferguson, DO
Co-editor: Donna Smith, PhD
Managing Editor: Kristine Pasciak
©2024 Medical Review Officer Certification Council
ISSN: 2833-0870
MRO Quarterly is an educational publication intended to provide information and opinion to health professionals. The statements and opinions contained in this document are solely those of the individual authors/contributors and not MROCC. MROCC and its editorial staff disclaim responsibility for any injury to persons or property resulting from any ideas or products referred to in this newsletter.
To unsubscribe from MROCC emails, please send an email to mrocc@mrocc.org with the subject unsubscribe.
