Impact of Δ8-THC on Marijuana Confirmation Rates in Non-Regulated Urine Samples
INTRODUCTION
Designer cannabinoid use is prevalent in the United States with sales of various designer or modified THC products available in gas stations, grocery stores, and pharmacies. These products were developed to circumvent the federal testing policy for natural plant marijuana as a Schedule I drug. The origins of these modified molecules started with the Hemp Bill of 2018 which allowed the extraction of CBD (Cannabidiol) from hemp (Δ9-THC content ≤ 0.3%) for its reported health benefits. The federal regulations specify Δ9-THC metabolite, often referred to as Carboxy-THC as a Schedule I drug, whereas designer molecules are commonly referred to as "Legal Weed".
CBD Production
The USDA 2021 hemp acreage statistics placed the value of hemp at $824 million with 84 square miles (54,200 acres) planted. Typically, 1,500-3,000 hemp plants can be grown per acre which is based on space and UV light requirements for proper plant development. CBD yields are typically one pound per hemp plant with a projection of at least 54 million pounds. Farmers found hemp crops to be far more lucrative with profits of $250-$300 per acre vs soybean of $30 per acre in Kentucky.
For example, a typical adult dose is approximately 50 mg per day. Based on this assumption, this results in a minimum of 50 billion doses based on the 2021 USDA statistics. The consumption of CBD did not keep up with the excess production. Therefore, marijuana chemists used the oversupply to create new compounds for additional profits and claimed legal status as the molecule is sourced from CBD. The legal status of the designer cannabinoids remains a grey area under federal law.
Δ8-THC Creation
The internet is filled with chemistry recipes to make Δ8-THC from CBD. It is a relatively straight forward chemistry project using concentrated acid to create Δ8-THC and Δ9-THC in a flask. With some creativity, large amounts can be created using a fermentation-type process similar to what is used for beer production. Since concentrated acid is used there must be a purification process to make an acceptable product for sale. It then can be converted into gummies, vapes, lotions, etc. for sale.
Δ8-THC Impact to Drug Testing
The initial use of the Δ8-THC was observed by CRL in March of 2020 with 4% of our marijuana confirmation samples containing substantial amounts of Δ8-THC metabolite. By July 2022, Δ8-THC was found in 33% of marijuana confirmation samples. The increasing prevalence of the Δ8-THC metabolite suggests that the use of the alternative Δ8-THC products are increasing and the promotion of "Legal Weed" is taking hold.
In April 2023 CRL began testing for both Δ8-THC and Δ9-THC metabolites in all marijuana confirmation tests. An analysis of the initial data was conducted in August with 29,847 screen positive samples. The highest value for Δ8-THC metabolite was 33,067 ng/mL in a Reasonable Cause sample and 50,949 ng/mL for Δ9-THC metabolite in a Pre-Employment sample.
Three charts have been created to digest this information. Chart #1 displays the breakdown by "Reason for Test" with Pre-Employment consisting of 52% of the tests. The chart further breaks the data into other testing categories.

Chart #2 is a further percentage breakdown of the Reason for Test, but each category is further broken down into those samples which tested positive for Δ9-THC above the 15 ng/mL cutoff (blue bars), the next category contains the percentage of samples with Δ9-THC below 15 ng/mL but contains Δ8-THC metabolite above 15 ng/mL. The grey and yellow bars are those samples which did not exceed 15 ng/mL but separated by those that contain some Δ8-THC and those which did not.

Chart #3 is a summary of all samples by Reason for Test based on the separation of concentrations listed above. Only 81.6% of all screen positive samples for marijuana are now confirming at the 15 ng/mL cutoff. This means that 18.4% of the screen positive samples are no longer confirming due to the ingestion of Δ8-THC or other cannabinoids. Although a couple of drugs are known to cause a marijuana positive screen (Protonix and HIV drug), it does not account for 6.3% of the samples being completely negative.
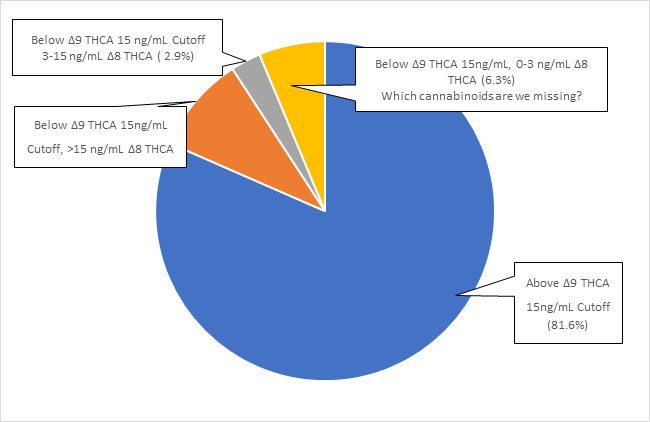
Historically, the confirmation rate for marijuana samples was greater than 99% and now because of alternative THC products has fallen to 81.6%. The immunoassay screen used in the laboratory has not changed which means alternate cannabinoids are being used that the laboratory is not detecting. CRL purchased reference standards to begin searching for two additional "designer cannabinoids" in urine and oral fluids. They are HHC (Hexahydrocannabinoid) and Δ10-THC. Both are widely sold and promoted in stores and the internet, however, some of the designer compounds may only be 50-90% as effective as Δ9-THC. The desired effects are adjusted by dose taken. There are others such as THCP (Tetrahydrocannabiphorol) which is reported to be 33-times stronger than traditional plant marijuana in its ability to interact with the brain CB1 receptor. Both THCP and THC-O-Acetate are known to create "psychedelic-like" effects.
Summary
Designer cannabinoids are chemistry project cannabinoids that trace their beginnings to the Hemp Farm Bill for the production of CBD with purported health benefits. Within the last month, HHS made the recommendation to move marijuana from Schedule I to Schedule III, the same Schedule as Tylenol #3. How do we regulate this as a pharmaceutical product? Pharmacies are controlled by a state board of pharmacy without any experience in this area. How do we regulate?
Perhaps nothing will change for the foreseeable future. After all, this will require the DEA and Congress to find a pathway forward, but the marijuana industry will spend huge amounts of money for changes.
INSIGHTS INTO THE DOT ORAL FLUID FINAL RULE FOR 49 CFR PART 40 AND ITS IMPACTS ON MEDICAL REVIEW OFFICERS ON MATTERS OTHER THAN ORAL FLUID TESTING
Senior Policy Executive Advisor, National Drug & Alcohol Screening Association
Mandatory Disclaimer: I am an employee of the United States Department of Transportation (DOT) on detail to the National Drug and Alcohol Screening Association (NDASA) as the Senior Policy Executive Advisor. The views reflected here are expressed in my personal capacity. My views have not been subjected to review, clearance, or approval by DOT, and they do not necessarily represent the views of DOT.
In the last issue of this newsletter, Dr. Donna Smith covered many elements of the DOT's Oral Fluid Final Rule, 88 Federal Register 27596 (May 2, 2023), which became effective on June 1, 2023. In this article, we will dig deeper into some of the DOT's final rule changes that are not part of the implementation of oral fluid drug testing but will impact MROs. The 417 commenters to this DOT rulemaking including MROCC and individual MROs, submitted excellent comments that provided insight, guidance, and affirmation that caused changes to the final rule. The result was a stronger final rule with a better rationale for the changes made. Here are some of the relevant provisions:
Use of State and Federal IDs instead of Social Security Numbers
In 49 CFR § 40.3, DOT expanded the forms of identification that can be used to identify donors not employed as FMCSA-regulated drivers. Restricted by the term "SSN or Employee ID No." because it is the term the Department of Health and Human Services uses on the CCF, DOT has revised its definition to allow a SSN, an employee ID number, or any other State- or federally issued identification number to fulfill the part 40 requirement for a unique identification number. 88 Federal Register 27605.
Error correction training when the specimen was damaged in transportation
DOT amended 49 CFR § 40.33 to clarify "damage to a specimen resulting in it being cancelled does not require retraining of the collector, unless the error actually occurred during the collection process. When a specimen is damaged by a delivery truck, sort facility, or other part of the transportation process, or is lost in transit, it is not the result of an error by the collector during the collection process." So, the collector is not to be retrained for errors outside the collection process. 88 Federal Register 27611
No more collection site call centers!
DOT recognized that many MROs were cancelling tests when they were unable to get the missing copies of the Federal Drug Testing Custody and Control Form (CCF) because they could not reach the collector or their supervisor. Often MROs ended up contacting a general call center that made them wait days or weeks to reach the collector who had failed to send the needed paperwork such as CCF Copy 2 or a memorandum to correct an error.
Consequently, DOT changed 49 CFR § 40.40 to require the collector to provide the telephone number where they and/or their supervisor can be reached directly and promptly. The regulation now requires the telephone number provided on the CCF to provide direct access to the specific collector and/or their supervisor during the collection site's business hours. The collector must never provide a number for a call center. DOT noted, "Ensuring the MROs and their staffs have timely access to the collectors is likely to result in fewer cancellations. So, this is effectively a two-pronged approach to addressing the cancellation problem."88 Federal Register 27606-07.
MRO addresses must not be simply a post office box
DOT turned a long-existing interpretation into a regulatory provision at 49 CFR § 40.40(c)(2). On Step 1.B. of the CCF, the MRO must provide an address to a place of business and not simply a post office box. 88 Federal Register 27603.
MRO staff can now contact the pharmacy
To increase efficiency, lower costs, and balance MRO office workflow, DOT now allows MRO staff to contact the pharmacy to authenticate a prescription the donor has offered to the MRO. (49 CFR § 40.141(b)). No part of the MRO's verification interview of the donor has changed, only the subsequent checking with the pharmacy to authenticate the prescription.
The regulation requires the MRO to maintain oversight of this new staff function. MROs should outline or script what the staff will ask the pharmacy and occasionally monitor these calls for quality control.
In its public comments, ACOEM anticipated MRO staff would have difficulty speaking with pharmacies due to HIPAA restrictions. DOT agreed, noting that while HIPAA does not apply to MROs' functions in DOT-regulated drug testing, it does apply to pharmacists providing healthcare services. If a pharmacist will not speak with the MRO staff, the MRO staff should contact the donor to authorize the pharmacist to communicate the information needed to verify the authenticity of the prescription. If the donor does not do this, then the MRO must report the verified non-negative result because the MRO could not authenticate the prescription. In other words, the donor did not provide a legitimate medical explanation that could be authenticated. There is a more extensive discussion of this in 88 Federal Register 27620-21.
Direction to MROs to reject any test not conducted under Part 40
Frequently, MROs are pressured by donors to accept a drug test the donor took after the DOT test, to show they had a negative result (of course). DOT wanted to provide MROs with a regulatory provision they could cite to donors to explain why the MRO must not consider the results of such additional testing. The regulatory language now says, "You must not consider any evidence (verbal or written information) from any drug tests that are not collected or tested in accordance with this part." 49 CFR § 40.151(a) For additional technical changes to 49 CFR § 40.151, see 88 Federal Register 27621.
MRO Stamp versus signature
In 49 CFR § 40.163(e), DOT clarified the records the MRO must retain after reporting a result. DOT emphasized the MRO must sign and date CCF Copy 2 for non-negatives, and the MRO staff can affix the MRO's stamp to and date Copy 2 for negatives only.
In the next edition of this newsletter, we will address the DOT's changes that will impact MROs who review oral fluid results.
MROs can reverse a test they cancelled
In 49 CFR § 40.207(d), the DOT now has given the MRO the ability to "uncancel" a test the MRO cancelled due to a correctable flaw, which was not corrected before the MRO sent the cancellation to the employer. Correctible flaws are specifically found in 49 CFR §§ 40.203 and 40.205. For example, if the MRO cancels a test due to missing paperwork that later appears after the cancellation (for example, when the collection site finds out they will not be paid for the collection without the paperwork), the MRO can now reverse the cancelled test and report the verified result to the employer. Although some MROs commented they were already doing this, it was never permitted under Part 40 until the June 1 effective date for the oral fluid final rule.
If the MRO wants to reverse a cancellation more than 60 days after initially cancelling the test, the MRO must consult ODAPC. If this seems familiar, it is because it is similar to the requirement in 49 CFR § 40.149(a)(4) for the MRO to consult with ODAPC to reopen a verified test after 60 days.
It is important to note the new regulation does not allow an MRO to reverse the cancellation of a test that was rejected for testing by a laboratory. When a laboratory has cancelled a test result or there are substantive grounds for the cancellation, it cannot be reversed. Specifically, cancellations for the following reasons must not be reversed: 49 CFR § 40.133 (verifying an invalid result without a donor interview), 49 CFR § 40.145 (a legitimate explanation for an adulterated or substituted result), 49 CFR § 40.159 (a specific explanation for an invalid result), 49 CFR § 40.161 (laboratory rejection of a fatal flaw or an uncorrected flaw); 49 CFR § 40.187 (the split fails to reconfirm or bottle B is unavailable for testing); 49 CFR § 40.191 (a refusal to go for a medical examination where there is no contingent offer of employment on a pre-employment test); 49 CFR § 40.193 (an acceptable medical explanation for an insufficient specimen); 49 CFR § 40.195 (a medical examination reveals clinical evidence of drug use), and 49 CFR § 40.199 (the laboratory reports a fatal flaw). For more information, see 88 Federal Register 27606-07.
How to cancel without Copy 1 or 2
Several commenters noted that an MRO could not cancel a test without having CCF Copy 1 and Copy 2, per 49 CFR §§ 40.129(b), 40.161(a) and (c). They suggested "if the reason the MRO is cancelling the test is because the CCF paperwork is missing, then part 40 should allow the MRO to cancel the test without holding either or both Copies 1 and 2 of the CCF." 88 Federal Register 27607
DOT agreed and in 49 CFR § 40.129(b) struck the words "test cancelled" so that cancelled tests do not require both Copies 1 and 2, as verified non-negative results would require. They also amended 49 CFR §§ 40.161(a) and (c) to permit an MRO to use either copy or to issue a report, if Copy 1, Copy 2, or both are missing. 88 Federal Register 27607
Xylazine: The Unregulated Menace - How It's Changing the Drug Landscape
JANETTA BRYKSIN, PhD, DABCC, FADLM*
Emory University School of Medicine
*Corresponding author
Xylazine, originally formulated by Bayer Pharmaceuticals in 1962, is a tranquilizer, analgesic, and muscle relaxant primarily intended for veterinary applications. Notably, its usage has not received approval from the Food and Drug Administration (FDA) for human medical purposes due to its potent hypotensive and central nervous system depressant attributes1.
Despite its limited authorization, xylazine's accessibility has led to its widespread prevalence across the United States, frequently identified by the street name "tranq"2. Alarming reports have indicated its use as an adulterant in illicit substances, most notably in conjunction with fentanyl, to augment sedative effects3. Data from the Drug Enforcement Administration (DEA) highlights a notable increase in xylazine prevalence, particularly in the southern regions, while overdose fatalities associated with xylazine have been primarily concentrated in the northeastern United States4. While these reports may not encapsulate the entirety of xylazine-related cases, they collectively portray a distinct pattern, reminiscent of the spread of fentanyl. Both substances have shown an initial presence in the Northeast, followed by dissemination into the South and subsequently into the Midwest and West4.
Xylazine functions as a potent agonist of the central α-2-adrenergic receptor and exhibits toxidromes that mimic those of clonidine and tizanidine, given their shared mechanism of action5. Effects attributed to xylazine usage encompass dry mouth, somnolence, hyperglycemia, hypotension, bradycardia, miosis, hypothermia, coma, and respiratory depression5. The similarity in toxidromes complicates the distinction between opioid and xylazine overdoses. When combined with opioids, xylazine can exacerbate respiratory depression during overdose events. A study conducted at the University of Maryland reported that 78% of urine specimens testing positive for xylazine also contained fentanyl. Furthermore, cocaine, THC, and amphetamines were each detected in 57% of fentanyl-positive specimens, with morphine present in 44%. Notably, 87% of xylazine-positive specimens tested positive for two or more of these additional drugs, and 31% contained four or more of the five drugs6.7. However, it is crucial to emphasize that naloxone, an opioid receptor antagonist, is ineffective in reversing xylazine overdose since xylazine does not belong to the opioid class. Currently, there exists no FDA-approved medication or antidote for countering xylazine overdose in humans, necessitating a reliance on supportive care measures, including assisted respiration and blood pressure management8.
Repetitive exposure to xylazine, whether through injection or intranasal administration, has been associated with severe skin and soft tissue injuries that may escalate to the point of requiring skin grafts or amputation9. These cutaneous ulcerations are notoriously challenging to treat and can rapidly extend to regions distant from the original site of administration, serving as diagnostic indicators of xylazine utilization. Additionally, evidence points to the development of severe dependence and withdrawal symptoms following repeated exposure to xylazine. The Philadelphia Department of Health has noted that inpatient treatment for opioid withdrawal may be considerably more complex when compounded with xylazine withdrawal, requiring supplementary pharmacological interventions if patients are simultaneously experiencing xylazine withdrawal. Early management of opioid withdrawal symptoms can alleviate potential discomfort that might exacerbate xylazine withdrawal10. Experts in addiction medicine and toxicology advocate the use of benzodiazepines and/or α-2-adrenergic receptor agonists, such as clonidine, dexmedetomidine, and tizanidine, to manage these co-occurring withdrawal syndromes11-13.
At present, a comprehensive nationwide assessment of xylazine-related overdose fatalities remains elusive, primarily due to its unregulated status. This same regulatory oversight also results in infrequent testing for xylazine in toxicological and forensic laboratories, thus contributing to a significant underestimation of xylazine misuse prevalence. However, alarming trends have emerged, with xylazine-positive overdose deaths increasing over 600% from 2020 to 2021, as reported by the DEA4. Recognizing the uncontrolled proliferation of xylazine and its emergence as an illicit street drug, at least seven states in 2022 have taken decisive actions by regulating or scheduling xylazine as a controlled dangerous substance. Furthermore, the FDA issues an important notice regarding the unlawful use of xylazine in February 2023, and the federal government designated the combination of fentanyl with xylazine as an emerging threat to the nation in April 2023. It is anticipated that more states will follow as they observe its misuse spreading.
Efforts to improve the detection of xylazine in drugs and human specimens have also gained traction. Harm reduction groups across the nation began providing test strips to check drugs for the presence of xylazine. These strips are generating acceptable results for drug checking purpose14. Because any drug obtained illegally now comes with the roulette of contamination. These initiatives aim to empower users to make informed choices about their substance use and reduce the associated risks. Public health laboratories and private testing companies, like NMS and Labcorp, have developed xylazine tests in urine or other matrices by using liquid chromatography tandem mass spectrometry (LC-MS/MS).
The public needs to understand the increased dangers posed by xylazine on the street. Therefore, exercising extreme caution in all illicit drug encounters is essential until supply chains can be secured. Knowledge, detection, and avoidance of xylazine may very well be the difference between life and death in the current landscape of illicit substance use.
REFERENCES
- Administration, F.a.D., FDA warns about the risk of xylazine exposure in humans. 2022.
- Shobha Thangada, P.H.A.C., 2; Sarah Ali, MPH3; Jacqueline Nunez, MD4; James R. Gill, MD4; Robert F. Lawlor3; Susan B. Logan, MS, MPH, Notes from the Field: Xylazine, a Veterinary Tranquilizer, Identified as an Emerging Novel Substance in Drug Overdose Deaths - Connecticut, 2019-2020. Morbidity and Mortality Weekly Report (MMWR), 2021. 2021(70): p. 1303-1304.
- Nunez, J., M.E. DeJoseph, and J.R. Gill, Xylazine, a Veterinary Tranquilizer, Detected in 42 Accidental Fentanyl Intoxication Deaths. Am J Forensic Med Pathol, 2021. 42(1): p. 9-11.
- Administation, D.E., The growing threat of xylazine and its mixture with illicit drug. 2022.
- Mohr ALA, B.T., Martin D, Logan BK, Xylazine: A toxic Adulterant Found in Illicit Street Drugs: U.S. Dept of State. 2020.
- University of Maryland Center for Substance Use, A.H.R., Emergency Department Drug Surveillance (EDDS) System: Summary of Initial Xylazine Findings. 2023.
- Holt, A.C., et al., Widespread Distribution of Xylazine Detected Throughout the United States in Healthcare Patient Samples. J Addict Med, 2023. 17(4): p. 468-470.
- Ruiz-Colon, K., et al., Xylazine intoxication in humans and its importance as an emerging adulterant in abused drugs: A comprehensive review of the literature. Forensic Sci Int, 2014. 240: p. 1-8.
- Malayala, S.V., et al., Xylazine-Induced Skin Ulcers in a Person Who Injects Drugs in Philadelphia, Pennsylvania, USA. Cureus, 2022. 14(8): p. e28160.
- Health, P.D.o., Health alert: risks of xylazine use and withdrawal in people who use drugs in Philadelphia. 2022.
- Capraro, A.J., J.F. Wiley, 2nd, and J.R. Tucker, Severe intoxication from xylazine inhalation. Pediatr Emerg Care, 2001. 17(6): p. 447-8.
- Elejalde, J.I., et al., Drug abuse with inhalated xylazine. Eur J Emerg Med, 2003. 10(3): p. 252-3.
- Ehrman-Dupre, R., et al., Management of Xylazine Withdrawal in a Hospitalized Patient: A Case Report. J Addict Med, 2022. 16(5): p. 595-598.
- Krotulski, A.J.S., J; Debord, J; Teixeira de Silva, D; Logan, BK., Evaluation of Xylazine Test Strips (BTNX) For Drug Checking Purpose. 2023.
Legalization of Cannabis - Implications for Workplace Safety
Reprinted with permission from the American College of Occupational and Environmental Medicine (ACOEM). ACOEM Task Force on Cannabis. ACOEM Position Statement: Legalization of Cannabis - Implications for Workplace Safety. August 17, 2023. Available at: https://acoem.org/acoem/media/News-Library/Position-Statement-Cannabis-8-31-2023.pdf.
The American College of Occupational and Environmental Medicine (ACOEM) is the largest international medical society representing occupational and environmental medicine (OEM) physicians and associated health care professionals. The College provides leadership to promote optimal health and safety of workers, workplaces, and environments.
Cannabis has the capacity to impair neurocognitive and psychomotor function, and its legalization has huge public health implications. Before Congress passes any legislation regarding cannabis, the College urges that the impact of such legislation on workplace safety be considered. To date, the house of medicine has not addressed the impact of cannabis on workplace safety.
Employers have a legal responsibility to protect employees from workplace illness or injury under the Occupational Safety and Health Administration's general duty clause. Employers also have an ethical responsibility to prevent impaired workers from exposing themselves, their co-workers, and/or the general public to risk of harm. Regardless of cannabis' legal status in a jurisdiction, ACOEM strongly supports the need for employers to prohibit all workers, especially those employed in safety-sensitive positions, from working impaired, whether under the influence of cannabis or any other potentially impairing substance. One issue is that there is no agreement as to the definition of working impaired.
To date, 38 states and the District of Columbia have legalized the medical and/or adult use of cannabis. With most Americans living and working in states that allow some form of legal cannabis use, it is critical that safety be at the forefront of any policy discussions regarding the use of cannabinoids. The current patchwork of laws and regulations to address the use of cannabis products is inconsistent and can be confusing and challenging to employees and employers in addressing workplace safety. Unlike Federal Drug Administration approved medications which have identified active component(s), a known side effect profile, a scientifically identified half-life and specific dose and dosing interval, these characteristics are not identified nor consistent between cannabis products.
ACOEM unambiguously states that cannabis products have the capacity to negatively affect judgment, motor coordination, reaction times, and driving ability. The problem is that to date there is no consistent definition of a dose or blood concentration of cannabis at which impairment begins or ends. Taking that into account, urine testing is not a measure of impairment, it is only an indicator of past use. Although there are ways to measure impairment, well-validated tools are not readily available or easy to implement in the workplace and at present are more useful in research settings.
ACOEM is advocating for increased research and collaboration between all stakeholders in defining and/or measuring the impairment risks of cannabis products use as it relates to the workplace. This would include the development and implementation of scientifically based methods for monitoring and testing for impairment from any of the myriad of substances that can cause it ( cannabis, alcohol, illicit drugs, opioids, or other medication whether prescript or over-the-counter).
Until the research is able to identify well-validated methods to evaluate and predict impairment caused by cannabis products, employers should establish workplace policies that address the following principles:
- The consequences of being at work while impaired.
- Specifically states whether use of cannabis products or any other potentially impairing substance should or should not be permitted while an employee is on duty. In making this decision the employer should consider whether the associated impairment will pose a risk to the employee, coworkers, or the public. This includes assurance of safe transport to and from work.
- Workers who are suspected of being impaired as a result of use of cannabis products or any other substance or condition should be subject to further evaluation including, depending on the circumstances, prompt removal from the workplace.
- Employers should be permitted to prohibit employees in safety-sensitive jobs from using potentially impairing cannabis for a minimum specified period ( at least 8-12 hours) prior to reporting for work as well as while at work, to ensure that they are not impaired upon arrival or during the workday.
ACOEM recommends the following areas be considered as part of the process in developing new cannabis legislation:
- Assess the impact of cannabis on workplace safety through research.
- Characterize, in detail, the range of challenges for employers posed by the differences between state and federal laws regarding cannabis product use. Identify options for reconciling these differences.
- Allow employers to manage the risk of adverse events in the workplace while knowledge of impairment lags behind the rapidly expanding use of cannabis and other cannabinoids.
- Evaluate means of ensuring safety while recognizing that the effects of cannabis products can vary greatly. Factors, including drug potency, the route of administration (inhalation, oral, sub lingual, or topical), and individual physiology and tolerance to cannabis greatly affect the magnitude, onset, and duration of impairment.
- Identify a reliable, practical evidence-based behavioral observation or a combination of tools for employers to assess fitness for duty by detecting impairment and/or by defining a per se level.
- Until the science of impairment by cannabis is better defined, a reasonable approach is to set a period of time between the use of cannabis and performing safety-sensitive work.
- Evaluate the difference in impairment characteristics between the occasional and regular user. What is the risk that regular cannabis use results in chronic low-level impairment in the absence of acute consumption? To what extent do chronic cannabis users exhibit tolerance to impairment? Do they return to a "normal baseline"?
As previously stated, except where specified by law, the employer has the primary responsibility to ensure the safety of its employees and the general public. Employers are the ones best suited to determine
if a job is safety sensitive and, should define "safety sensitive" in their workplaces. ACOEM proposes positions be classified as safety sensitive if they involve the use of firearms, emergency response, and/
or judgment and decision-making that have an immediate impact on the life and health of others. Or, if impairment while performing job duties would:
- Increase safety and health risks to self, fellow employees, contract personnel, or the public;
- Adversely affect the environment through contamination of air, water, soil, flora, or fauna;
- Jeopardize the community through property damage or by endangering members of the public.
As the leading OEM medical association, ACOEM is ready to assist policy makers at all levels of government in addressing the implications of cannabis legislation on workplace and environmental safety.
Government Resource Websites
ODAPC - DOT
- Office of Drug & Alcohol Policy & Compliance (ODAPC)
- DOT 49 CFR Part 40 Procedures for Transportation Workplace Drug and Alcohol Testing Programs
- Subscribe to the ODAPC Updates and News
- 2023 DOT Random Testing Rates
SAMHSA - HHS
- Medical Review Officer Guidance Manual for Federal Workplace Drug Testing Programs
- Mandatory Guidelines for Federal Workplace Drug Testing Programs using Urine (UrMG)
- Mandatory Guidelines for Federal Workplace Drug Testing Programs using Oral Fluid (OFMG)
NRC
CUSTODY AND CONTROL FORMS (CCFs)
- 2020 Federal CCF for Urine and Oral Fluid Specimens
- 2020 Guidance for Using the Federal CCF for Urine Specimens
- 2017 Federal CCF for Urine Specimens - accepted for urine specimens until August 31, 2023



