Introduction to Alcohol Biomarkers
Vault Health
Alcohol is still the most abused substance in the world. It is legal and as such testing for it in workplace settings is appropriately limited to attempts to determine impairment according to existing legislative and workplace policy requirements. MRO training courses also limit instruction for alcohol testing to impairment, however some MROs find themselves asked to review more advanced testing protocols like those used in monitoring, return to duty, follow up and last chance testing. Donors in those programs are required to completely abstain from any alcohol ingestion, so those programs test to determine abstinence rather than impairment by testing for alcohol biomarkers rather than blood or breath alcohol levels. Biomarkers are indicators of past ingestion, not impairment and the ability to review them is necessary for MROs tasked with verifying program compliance and even possible relapse.
Ethyl Glucuronide (EtG), Ethyl Sulfate (EtS), and Phosphatidyl Ethanol (PEth) are direct biomarkers or metabolites of ethanol. They are termed "direct" because they are direct products of the metabolism of ethanol, and no other alcohols metabolize to them. Although most alcohol that is consumed is metabolized by oxidative processes in the liver, a very small amount is broken down non-oxidatively, thereby creating these biomarkers that can be measured for longer than ethanol itself remains detectable. EtG and EtS are usually measured in urine specimens. PEth can be measured either in whole blood or blood spot testing.
EtG/EtS concentrations generally represent about 0.02%-0.06% of total ethanol elimination. Published literature indicates that EtG may be detectable for up to 80 hours after alcohol ingestion. The EtG and EtS "windows of detection" are dependent on cut-off levels used, individual metabolism, alcohol usage patterns, and the concentration of the urine specimen being tested.
The presence of EtG and EtS in urine specimens is confirmed using LC/MS/MS methodology. The cutoff level for EtG confirmation may be 100 ng/mL, 250 ng/mL or 500 ng/mL or higher; the EtS confirmation cut-off level is usually 75-100 ng/mL.
Because of the sensitivity of both EtG and EtS testing it is possible for exposure to alcohol from use of personal hygiene products, foods containing alcohol, and cleaning or sanitizing products to result in a positive EtG and/or EtS result. EtG/EtS testing cannot absolutely distinguish between beverage alcohol consumption and incidental or unintentional exposure from these ethanol containing sources based on the EtG/EtS levels alone. It is strongly recommended that low level EtG/EtS results be interpreted with consideration of non-drinking exposure to alcohol. It is also strongly recommended that MRO review be considered for all EtG/EtS positive results.
Recent studies have indicated that EtG can be either formed or degraded in a urine specimen when certain conditions are present. EtG is subject to degradation by some bacteria at room temperature. Also, under certain conditions, in-vitro formation of EtG may occur in the specimen container when certain bacteria and ethanol or ethanol-producing bacteria are both present in a urine specimen. Because of these two factors related to EtG degradation and in-vitro production, it is strongly recommended that EtS testing always be conducted in conjunction with testing for EtG. Unlike EtG, EtS is completely stable once it is formed, and it is formed usually within 24 hours after ingestion. There are no published reports of in-vitro synthesis of EtS or degradation of EtS in urine specimens. Additionally, urine specimens being testing for EtG/EtS should arrive at the testing laboratory within 5 days of specimen collection to minimize EtG level changes. A word of warning when reviewing EtG/EtS results: Do not attempt to use EtG/EtS ratios to determine the time of ingestion. There are too many variables at play to make those ratios forensically defensible.
EtG and EtS are most reliably measured in urine specimens. Recently hair and oral fluid have been used as alternative matrices to measure these biomarkers, but urine is still the most researched and preferred matrix. Because of the chemical composition of the hair shaft, only EtG can be tested in hair, not EtS. There is very little peer reviewed data that support EtG testing in oral fluid, especially oral fluid Point of Collection Testing (POCT).
Testing for EtG/EtS got off to a bit of a rocky start. Initially positive EtG levels were considered to be synonymous with drinking and unfortunately that concept was sold by some laboratories and testing programs. It wasn’t long before science reared its head and indicated that was a false precept. Urine testing for EtG and EtS is very sensitive, arguably too sensitive, and positive results can be obtained from both innocent dermal absorption of ethanol from things like hand sanitizers or ingestion of food prepared with alcohol. This controversy brought about a “Black Box Warning” in 2006 from SAMHSA’s Center for Substance Abuse Treatment (CSAT) which was not revised until July 2012 when an updated Advisory was published which noted:
Because of the common use of EtG to document abstinence in various settings and the grave consequences for false positive, much attention has been given to the cutoff values of EtG. Although further research is needed before firm cutoffs for EtG can be established, sufficient research has been completed to reach the following conclusions:
A "high" positive (e.g., > 1,000 ng/mL) may indicate:
- Heavy drinking on the same day or previously (e.g., previous day or two)
- Light drinking the same day
A "low" positive (e.g., 500–1,000 ng/mL) may indicate:
- Previous heavy drinking (previous 1–3 days)
- Recent light drinking (e.g., past 24 hours)
- Recent intense "extraneous" exposure (within 24 hours or less)
A "very low" positive (100–500 ng/mL) may indicate:
- Previous heavy drinking (1–3 days)
- Previous light drinking (12–36 hours)
- Recent "extraneous" exposure
Phosphatidyl Ethanol (PEth): While EtG and EtS testing can be effective tools to assist in alcohol abstinence testing, they should be used alone with caution. Many sources recommend the use of PEth testing either by itself or as a follow-up to non definitive EtG/EtS results because it can be a great help in differentiating between innocent or extraneous ingestion of alcohol and drinking. PEth is a blood test and has an average detection period of about 2-4 weeks depending on the amount and pattern of alcohol consumption. PEth was discovered in mammals in 1983. It is a group of phospholipids consisting of a glycerol backbone, phosphoethanol as headgroup, and 2 fatty acid moieties in sn-1 and sn-2 positions. It is formed in the cell membranes of red blood cells by means of phospholipase D. PEth is not one molecule but rather a collection of subspecies. Inter-individual differences in the relative abundances of PEth homologues do exist, but current data suggest that 5 molecular species (16:0/18:1, 16:0/18:2, 16:0/20:4, 18:1/18:1, 18:1/18:2) could constitute more than 80% of total PEth. Routinely tested species are 16:0/18:1 PEth, 18:1/18:1 PEth and 16:0/18:2 PEth.
PEth research has been published in peer reviewed literature since its discovery in 1983 and no false positives have been reported. The consumption of 60 gm of alcohol (3-4 six ounce glasses of wine) or more is currently considered the benchmark for the amount of ingestion that will cause a PEth positive at the 20 ng/mL cutoff, the most commonly used industry cutoff. Care should be taken when attempting to relate a PEth level with a specific amount of ingestion or time during the detection window that ingestion occurred. The length of the detection window makes that difficult to do accurately.
Incidental ingestion or dermal absorption of ethanol have not been shown to cause PEth positives as they might with urine EtG/EtS testing. Negative PEth tests when performed within the detection period after non definitive EtG/EtS positives are extremely helpful in supporting claims of abstinence. Conversely, positive PEth results performed as follow ups to those same EtG/EtS results can help to determine whether alcohol consumption actually occurred.
PEth, EtG and EtS are all laboratory developed tests (LDTs). There is no national oversight agency for any LDT so the selection of laboratories to perform these tests is critical. Only laboratories with the highest overall standards and certifications should be used. Given that, laboratory protocols may vary and laboratory results may differ. Care is recommended in the interpretation of all biomarker results, and a collaborative relationship with knowledgeable laboratory toxicologists is strongly recommended.
What is Delta-8-THC and Does it Interfere with the Drug Test?
Recently the New York Times published an article on delta-8-THC1. In that article they mentioned that Google searches for delta-8-THC grew more than 850% in the US between 2020 and 2021, particularly in states that have legalized marijuana. They also mentioned that 16% of regular marijuana users also use the delta-8 version.
Delta-8-THC is chemically similar to the delta-9-THC found in marijuana plants. The only difference is the position of a double bond and therefore these versions are considered to be structural isomers.
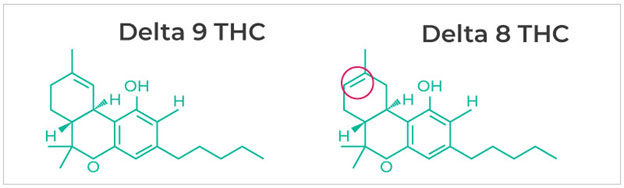
As with other isomers they both have pharmacological activity, although that activity varies. This is also the case with the stereoisomeric forms of methamphetamine and some narcotics. Both delta-8 and delta-9-THC bind to the CB1 receptor and other cannabinoid receptors in the brain, although the delta-8 isomer has a weaker attachment to the CB1 receptor. This means that it is less potent than the delta-9 isomer.
There is another structural isomer of THC and that is delta-10-THC and this also differs from the delta-9 version of THC in the position of the double bond. It is reportedly less potent than the delta-8 isomer. I am not going to discuss this isomer further.
Reportedly delta-8-THC is about half as potent as delta-9. In a survey of delta-8 and delta-9 users researchers from the University of Buffalo2,3 reported that “…results suggest that delta-8-THC may be equally effective for desired purposes of cannabis use and lower in undesirable or adverse effects.”
The increase in delta-8-THC use is a result of the passage of the Farm Bill4 that legalized the growing of hemp and defined hemp as containing no more than 0.03% of THC. Above that percentage it is considered to be marijuana. Hemp also contains large amounts of cannabidiol (CBD) and delta-8-THC can be easily synthesized from this cannabinoid. A Google search showed many different procedures to do this, and reference number 5 below is just one example. One major result of this synthetic pathway for the delta-8 isomer is that it is considered to be legal by the Federal Government as it is derived from a chemical found in a legal plant, i.e., hemp. If the delta-8-THC was from a non-legal plant source, it would fall under Schedule 1 and therefore be illegal.
Discover Magazine6 has provided a summary of whether delta-8-THC is considered legal by the states. The following states consider delta-8-THC to be legal and allow its purchase:
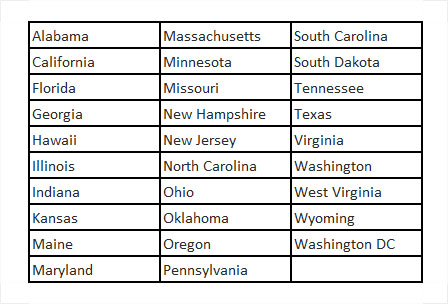
The following states consider delta-8-THC as recreational cannabis:

These states are classified as being in a “legal grey area”:
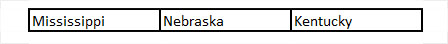
Finally, the following states consider hemp derived delta-8-THC to be illegal:

*A disclaimer – I have not personally verified each of the classifications above.
The second question is whether or not delta-8-THC and the carboxylic acid metabolite (nordelta-8-THC -carboxylic acid) are detectable after use in urine specimens. As we all know the drug test uses an immunoassay test for initial screening and a chromatography mass spectrometry test for confirmation, such as gas chromatography–mass spectrometry (GCMS) or liquid chromatography-mass spectrometry (LCMS or LCMSMS). In considering urine testing one would certainly expect the delta-8 metabolite to be cross-reactive in the immunoassay and therefore a presumptive positive urine specimen would be tested by the lab’s confirmation procedure for the delta-9 metabolite.
A group from the Karolinska Institute, Sweden published a paper in 20217 and they were able to separate the delta-8 and delta-9 metabolites by LCMSMS. In June 2020 the delta-8 metabolite was present in 5.3% of the specimens tested, but since then they only notice the occasional specimen.
An abstract presented at the 2022 AACC Meeting from Garg et al.8 showed that the delta–8 metabolite cross-reacted with the Siemens Emit II Plus cannabinoid immunoassay. They spiked negative urine with delta-8-THC metabolite at concentrations between 10 and 50 ng/mL and showed that using a cut-off of 20 ng/mL the assay detected delta-8 metabolite concentrations of 30 ng/mL and higher. They were able to separate the isomers using GCMS due to differing retention times for the two metabolites. As you would expect, fragmentation of the delta-8 metabolite resulted in the same MS fragmentation ions as the delta-9. They confirmed these findings in one urine sample containing both delta-8 and delta-9-THC metabolites.
As a point of information, the Department of Defense routinely confirms for the presence of the delta-8 metabolite in urine specimens collected from military members. I have seen no published data on the positive rates. They do, however, consider it a positive finding and subject to action under their program.
I could not find similar studies on the detection of the delta-8 and delta-9-THC in oral fluids. However, I would expect similar results – positive immunoassay and negative confirmation results for the delta-8 isomer.
Certainly, drug testing labs need to add the ability to separate the isomers to their validation studies for the cannabinoid metabolites in all specimen types they test. If they cannot be separated then the delta-8 isomer could give a positive result or contribute to a positive result assuming delta-9 is present also.
In summary delta-8-THC has similar pharmacological activity as delta-9-THC, however, it is less potent, it is considered “legal” if it results from a synthesis from CBD found in hemp and varying state consider it to be legal, urine specimens containing the delta-8 metabolite can test positive by immunoassay screening tests, however, these should confirm as negative.
REFERENCES
- Smith, D. (2022, July 25). How Delta-8 THC Works, and Why Experts Are Worried About It. New York Times. https://www.nytimes.com/2022/07/01/well/mind/delta-8-thc-marijuana.html
- Kruger, D. J., & Kruger, J. S. (2021). Consumer Experiences with Delta-8-THC: Medical Use, Pharmaceutical Substitution, and Comparisons with Delta-9-THC. Cannabis and Cannabinoid Research. https://doi.org/10.1089/can.2021.0124
- Kruger, J. S., & Kruger, D. J. (2022). Delta-8-THC: Delta-9-THC’s nicer younger sibling? Journal of Cannabis Research, 4(1). https://doi.org/10.1186/s42238-021-00115-8
- Hudak, J. (2018, December 14). The Farm Bill, hemp legalization and the status of CBD: An explainer. Brookings; Brookings. https://www.brookings.edu/blog/fixgov/2018/12/14/the-farm-bill-hemp-and-cbd-explainer/
- Mackel, K. (2021, March 29). How Delta-8 THC is Made in the Lab. Www.cannabistech.com. https://www.cannabistech.com/articles/how-delta-8-is-made-in-the-lab/
- Smith, A. (2022, September 20). Is Delta 8 THC Legal in Your State? Our Guide. Discover Magazine. https://www.discovermagazine.com/lifestyle/is-delta-8-thc-legal-in-your-state-our-guide
- Helander, A., Johansson, M., Andersson, A., & Villén, T. (2021). Analytical and medico‐legal problems linked to the presence of delta‐8‐tetrahydrocannabinol (delta‐8‐THC): Results from urine drug testing in Sweden. Drug Testing and Analysis, 14(2), 371–376. https://doi.org/10.1002/dta.3190
- Garg, U., Baird, S., & Frazee, C. (2022, July 26). A-302. Can Current Immunoassay and Gas-Chromatography Mass Spectrometry (GC-MS) methods for delta-9-tetrahydrocannabinol carboxylic acid (Δ9-THC-COOH) detect Δ8-THC-COOH? 2022 AACC Annual Scientific Meeting. https://www.abstractsonline.com/pp8/#!/10594/presentation/293
A Positive DOT Test in Commercial Pilots
A1A Aviation Medicine, Inc.
A positive DOT alcohol or drug test in a commercial pilot, whether random, for suspicion, or following an accident, has significant ramifications beyond that for others. A pilot loses his aviation medical certificate, and also airman’s (flying) certification. To regain medical certification he or she must comply with the Human Intervention and Motivation Study (HIMS) Program under the guidance of an FAA HIMS Aviation Medical Examiner (AME). The program includes complete abstinence from all substances (including alcohol and marijuana) documented by 14 or more random tests every 12 months and/or Soberlink (an alcohol Breathalyzer that transmits results to the HIMS AME).
Intensive Outpatient (IOP) or residential recovery programs are expected. Following successful completion, weekly aftercare, AA (Alcoholics Anonymous), NA (Narcotics Anonymous) and BOAF (Birds of Feather is AA for pilots) participation is expected. Pilots are expected to be monitored by several sponsors and the HIMS AME. A pilot’s specific requirements vary based upon diagnosis, history, and clinical course. Detailed information on the HIMS Program is available at the website (https://Himsprogram.com).
In stable recovery, with no evidence to suggest ongoing substance abuse/use, nor a psychiatric diagnosis, the FAA requires an intensive evaluation by a board-certified psychiatrist, and a large battery of neuropsychological testing. Both are preferably by FAA-trained practitioners. The above takes at minimum three months, but often 6 to 12 months. Assuming all is supportive, an aviation examination is accomplished by the HIMS AME who must also support return to the cockpit.
A package of documents is forwarded to the FAA for consideration that typically takes an additional 3 to 10 months for a decision. A Special Issuance is at the discretion of the Federal Air Surgeon. If granted, the pilot is medically qualified to resume flying. However, the pilot must continue HIMS Program requirements initially no different than those prior to the Special Issuance. Special Issuances are limited to six months, and reapplications require favorable reports and support of the monitoring HIMS AME.
Over the next seven years the pilot may be stepped down with lessening requirements. However, for the rest of his or her career a pilot must remain abstinent of all substances and continue to be seen by HIMS AMEs.
Unless the pilot is an employee of a major airline, the HIMS Program and requirements are expensive and typically not covered by medical insurance. Costs are $10,000 to $15,000 for the first year, not including the cost of the residential or IOP program, and then somewhat less for subsequent years.
The pilot also faces FAA enforcement action with “Emergency Revocation” of all pilot flight certifications. The pilot should consider engaging an aviation lawyer. Under the best of circumstances, if a ”Prompt Settlement” is reached, the FAA will allow the pilot to apply for recertifications in a year. This means retaking and passing each and every written and flight test to regain flying privileges, again at considerable cost and time. If the pilot wishes to legally contest the DOT test, recertification and flying will be delayed much longer than a year.
As stringent and costly as all of this is, the upside is 85 percent of pilots who participate in HIMS return to the cockpit. I have had the privilege of assisting pilots regain medical certification after positive DOT tests for more than a decade. It is rewarding to see them safely flying again, and they are extremely grateful.
FMCSA Drug Testing Data on CDL Drivers
WFQA, LLC Regulatory Compliance Officer
The largest component of DOT-regulated drug testing is in the trucking industry. The Federal Motor Carrier Safety Administration (FMCSA) regulates approximately 600,000 motor carriers and 5,000,000 commercial drivers (CDL holders). There are more MROs reviewing, interpreting and reporting FMCSA drug test results than all of the other DOT agencies combined (FAA, FRA, etc.).
Each year the DOT agencies (FMCSA, FAA, FTA, etc.) must obtain and publish Drug and Alcohol Management Information System (DAMIS) data compiled by the employers whom they regulate. Because of the large numbers of FMCSA-regulated employers (motor carriers) and employees (CDL drivers), the FMCSA gathers MIS data from a representative sampling of the trucking industry. The results of the DAMIS reports filed with the FMCSA for drug testing conducted in calendar year 2020 are presented in the table below. The results presented are based on a survey of all companies with over 1000 CDL drivers, and a random sample of companies with fewer than 1000 CDL drivers. The summary data provided in the table represent raw counts, based on information provided by the motor carriers to DAMIS. The chart below represents drug testing data from 3,917 motor carriers who had 1,200,812 CDL drivers.
Each DOT agency that requires workplace drug testing compiles the data from the DAMIS reports submitted to them and provides it to the DOT Office of Drug and Alcohol Policy and Compliance (ODAPC). The agencies also use this data to calculate the estimated positive usage and violation rates for random testing in order to set the minimum annual random testing percentage for their industry. Calendar year 2020 is the most recent year for which DAMIS data is available.
You can access the DOT agencies’ DAMIS information, year by year, on the ODAPC website at https://www.transportation.gov/odapc/DOT_Agency_MIS_Data.

Note: Prior to generating estimates of positive usage rates from the DAMIS data, FMCSA conducts further checks on the data, for internal validity and consistency, making adjustments, as necessary. In using the random testing data to calculate the estimated drug usage and refusal to test rates for determining the random testing rate for the subsequent year, the FMCSA uses weighted estimates. The survey weight assigned to each motor carrier in the annual sample is determined by the size group category from which the carrier is selected. As a result, estimates of positive usage rates, based on the information provided in this summary table may not match results published by the agency.
In January 2020, the FMCSA implemented its Drug and Alcohol Clearinghouse which requires MROs, motor carriers and other employers who have commercial motor vehicles and CDL operators to report positive drug tests and refusals to test on CDL holders. The Clearinghouse data for 2020 gives a more comprehensive picture of the actual number of positive drug tests and refusals to test than the DAMIS survey data. Since the DAMIS data is a representative survey of the motor carriers and their drivers, it does not capture all the FMCSA drug tests conducted in a given year, only those conducted by the motor carriers who were selected to submit their testing data to the FMCSA. The Clearinghouse positive and refusal to test data must be submitted by every employer conducting FMCSA drug testing and every MRO who verifies FMCSA drug test results. In 2020, MROs reported 45,822 positive drug tests to the Clearinghouse; that’s over 3.6 times more positive tests than submitted in the DAMIS data surveying almost 4,000 motor carriers. The Clearinghouse also received 7,803 refusals to test submitted by MROs and employers. The FMCSA DAMIS data for 2020 had a total of 3,046 refusals to test, 40% less than was reported to the Clearinghouse. Both the DAMIS and the Clearinghouse positive drug test data show that marijuana is the most frequently detected drug in FMCSA drug tests with over 60% of the positive results reported positive for marijuana. FMCSA Drug and Alcohol Clearinghouse summary data is provided monthly with year-to-date totals and can be found at https://clearinghouse.fmcsa.dot.gov/Learn#news-events.
Fentanyl and Workforce Drug Testing
BACKGROUND:
Fentanyl is an extremely potent – approximately 100 greater than morphine – synthetic opioid narcotic analgesic first introduced into clinical practice in 1963 as Sublimaze®. In the United States (U.S.), Fentanyl is classified as a Schedule II drug and is available as oral transmucosal lozenges (aka “lollipops”, Actiq®), effervescent buccal tablets (Fentora™), sublingual tablets (Abstral®), sublingual (Subsys™) and nasal sprays (Lazanda®), transdermal patches (Duragesic®), and injectable formulations. While Sublimaze® (fentanyl citrate) has been discontinued in the U.S., generic products are still available. The oral formulations are used primarily for breakthrough cancer pain; patches are intended to be used for chronic pain management; and injectable forms are used for potent analgesia and anesthesia.
Fentanyl is lipophilic and highly (80-85%) protein bound, has a rapid onset of action, and a short duration of effects. It rapidly accumulates in muscle and fat and is slowly released into the blood. Hepatic metabolism is primarily via the CYP3A4 system resulting in n-dealkylation to norfentanyl. Consequently, other drugs mediated by this system can significantly impact fentanyl metabolism and its pharmacokinetics. The half-life of fentanyl is reported to range from 3-12 hours which is significantly impacted by the route of administration and metabolism. Approximately 75% of an intravenous dose of fentanyl is excreted in the urine, mostly as metabolites (primarily norfentanyl) with under 10% being excreted unchanged. Typically, norfentanyl is detectable longer and at higher concentrations in urine than fentanyl.
USAGE DATA:
Based on data from IQVIA™ (formerly IMS Health) reported by the DEA, the number of fentanyl prescriptions dropped 38% (6.5MM vs. 4.0MM) between 2015 and 2018.
Prescription fentanyl is one component of fentanyl misuse– e.g., diversion or removal of the gel contents from patches followed by injection or ingestion. Currently, the leading source of fentanyl misuse and overdose deaths involves illicitly manufactured fentanyl (IMF). The National Survey on Drug Use and Health (NSDUH) asks only about the misuse of prescription forms of fentanyl and may underrepresent people using IMFs. Between 2015 and 2020 the self-reported past-year illicit use of fentanyl held steady at 0.1%. The data from the Centers for Disease Control (CDC) and the National Forensic Laboratory Information System (NFLIS) presents a different picture of fentanyl misuse. In the NFLIS data, fentanyl reports – which do not differentiate between prescription fentanyl and IMFs –remained steady between 2007 and 2013, with dramatic increases between 2014 and 2020. All regions of the U.S. exhibited year-over-year (YoY) increases, except for the Northeast which declined between 2019 and 2020. While the NFLIS data is reflective only of what the laboratories were testing for in each case – i.e., it is not true prevalence data – and may have been impacted by COVID-19 pandemic-related resource constraints, in 2020 fentanyl was the fourth highest identified drug and represented 9% of all drug reports in the NFLIS data. Moreover, it accounted for 59% of the narcotic analgesic reports. Data from the CDC provides additional insights on the rate of opioid-related deaths and the impact of fentanyl over the last 20 years (Figures 1 & 2).
FIGURE 1
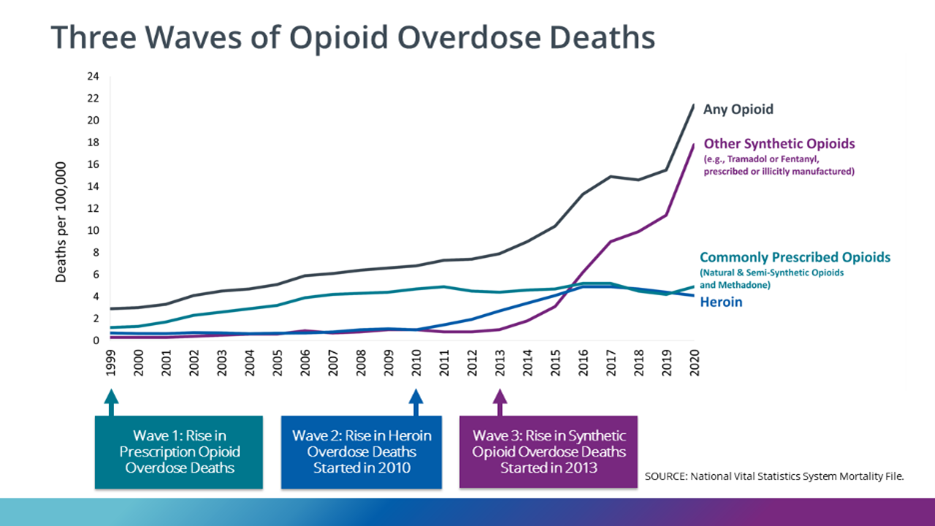
FIGURE 2
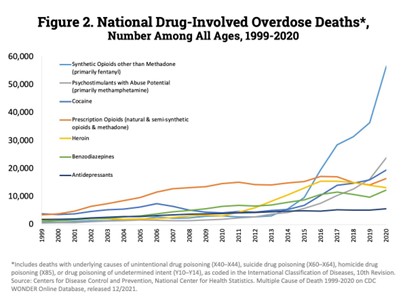
In this data, deaths related to synthetic opioids (primarily fentanyl) have risen dramatically since 2015. Between 1999 and 2020, overdose deaths due to Synthetic Opioids other than Methadone (primarily fentanyl) have exhibited YoY increases in all but 3 years (2007, 2011, 2012) with double digit increases every year since 2012. Fatalities increased 590% between 2015 and 2020 and are up more than 77-fold since 1999.
LABORATORY ANALYSIS:
Workforce testing for fentanyl use is uncommon but increasing. While the SUPPORT for Patients and Communities Act, which was signed into law in 2018, directs HHS/SAMHSA to determine whether fentanyl should be added to the Federal drug testing panel, the HHS is investigating its prevalence and laboratories readiness for testing. In non-regulated testing at two of the larger national labs, both have experienced a doubling of positivity rates in tests for fentanyl and/or norfentanyl – one lab has experienced a positivity rate increase from 0.18% (2017) to 0.39% (2021) and the other lab’s positivity increased from 0.31% (2019) to 0.67% (2022).
Today, urine testing for fentanyl commonly utilizes an immunoassay for screening followed by LC-MS/MS confirmatory analysis. There are three commercially available, FDA-cleared immunoassays. Two of these are targeted for fentanyl, while the third is targeted for norfentanyl. There are FDA compliant protocols for the fentanyl immunoassays at 0.5 and/or 1 ng/mL. The norfentanyl immunoassay utilizes a 5 ng/mL cutoff. The cross-reactivity for norfentanyl is relatively low (≤ 7%) in the fentanyl immunoassays. Interestingly, one these assays exhibits relatively efficient detection of norfentanyl even in the absence of fentanyl suggesting that there may be other unidentified fentanyl metabolites contributing to the overall response. ELISA immunoassays have also been marketed for the detection of fentanyl/norfentanyl. However, none are FDA-cleared or labelled as being for Employment and Insurance testing. Confirmatory urine testing for fentanyl typically measures both fentanyl and norfentanyl at a 0.5 or 1 ng/mL cutoff. There are also commercially available immunoassays, some of which are FDA compliant, for oral fluid and hair testing of fentanyl. In reviewing laboratory results for fentanyl, or comparing data from different labs, MROs should consult with the laboratory to determine the capabilities of their testing methodologies.
RESOURCES
- CDC: DOSE Dashboard: Nonfatal Overdose Data
- CDC: SUDORS Dashboard: Fatal Overdose Data
- DEA: Drug Chemical Information - Fentanyl
- DEA: NFLIS-DRUG 2020 ANNUAL REPORT
- NIDA: Overdose Death Rates
- NSDUH: 2020 NSDUH Annual National Report
Questions and Answers from ACOEM’s MRO Online Faculty Discussions
ACOEM’s virtual MRO Online course includes monthly live discussions with MRO faculty. Course participants are encouraged to submit questions for discussion. The following Q&As came from these discussions in early 2022. Readers of MROCC’s MRO Quarterly are welcome to submit questions to news@mrocc.org for possible inclusion in future editions of the newsletter and/or monthly discussions for ACOEM's virtual MRO course.
Answers were provided by the MRO faculty, Michael Peat, PhD, Kent Peterson, MD, and Donna Smith, PhD, with additional assistance from James Ferguson, DO and Douglas Martin, MD.
MEDICATION ASSISTED TREATMENT (MAT)
QUESTION: Can a Substance Abuse Professional (SAP) suggest to the patient that they would benefit from Medication Assisted Treatment (MAT), as part of their treatment and make a referral?
ANSWER: The SAP can suggest any treatment the SAP thinks is appropriate.
QUESTION: Am I correct in assuming that federal employees in safety-sensitive positions cannot be on methadone, but can be on Suboxone?
ANSWER: Methadone was prohibited by FMCSA but now it is considered to be in the same category as buprenorphine. Neither is permitted, but now there is also no absolute prohibition for driver certification. We recommend follow up directly with FMCSA if a case arises. Given that, guidance in the past has strongly suggested that examiners be very careful before they approve a CDL for a driver on buprenorphine.
QUESTION: Can any federal employee (DOT) be on Suboxone? Can CDL drivers in recovery from alcoholism be on Vivitrol if they are having no side effects?
ANSWER: Naltrexone administered in any form has no effect on federal drug testing, minimal side effects and should be no problem for safety-sensitive employees to be taking.
CALLS TO DONOR AND DER
QUESTION: When should the MRO call the donor and call the DER?
ANSWER: This question is addressed in the table below:

RE-COLLECTION OF URINE SPECIMEN UNDER DIRECT OBSERVATION
QUESTION: When should MRO ask for a repeat urine test and when should it include direct observation?
ANSWER: The MRO directs a re-collection of a urine specimen using direct observation procedures as follows:
- Test cancelled; no medical explanation for the laboratory invalid findings.
- Test cancelled: direct observation required, however was not performed and result was negative.
- Test cancelled: split specimen requested by donor, however no split specimen available for testing.
- Negative dilute result where laboratory reported creatinine 2-5 mg/dL.
In a pre-employment test circumstance where a “repeat test” is needed because the MRO reported a cancelled test, the “repeat test” is NOT directly observed unless one of the above conditions are met.
All return to duty and follow-up tests must be directly observed. So, if a return to duty/follow-up test is cancelled by the MRO it must be repeated as a direct observation collection.
OBSERVED URINE COLLECTIONS
QUESTION: Why doesn’t the donor have to pull up the pants legs to expose the lower leg and ankle such as is done when entering a proctored exam?
ANSWER: Since the “visual” inspection for urine specimen substitution/adulteration devices or paraphernalia is a prelude to the actual direct observation of urinating (watching urine go from body into the collection container), DOT felt that a device affixed to the leg or foot would have to be especially complex to deliver the substituted specimen or adulterant to the genital area where it would be undetected by the observer watching the person urinating. Certainly, there are several “cheating methods” that may not be detected by the DOT’s deterrent procedures, however additional detection methods (e.g. body cavity searches, full body inspections) were deemed too invasive and beyond the scope of the warranted search.
DILUTE URINES AND DETECTION OF ETG
QUESTION: How much would a very dilute urine sample prevent the pick-up of EtG? We are monitoring a physician with a severe Alcohol Use Disorder, and had received several very dilute urines, but negative for EtG. Because of our concern we started testing her via PeTH. The MRO did not report it as Invalid; however, the concern was that it would be possible to have such a dilute urine that EtG wouldn't be picked up at even a low concentration?
ANSWER: Because the lab is using a cut-off I would ask them to see if there is anything there below the cut-off. I also assume she has creatinine and specific gravity readings - - are they of any value? And is the lab looking for ethanol sulfate? Anything is always possible but it is hard to believe she always can beat the test.
If participant continues to produce dilute urines despite instruction to limit water intake in 2-3 hours prior to providing specimen, a blood test for PEth should be ordered.
ADDITION OF FENTANYL TO FEDERAL DRUG TESTING PANELS
QUESTION: It seems to me that, given the current state of the opioid epidemic, fentanyl should really be on the DOT panel. Why isn't it? Do you think the panel will be updated to include fentanyl?
ANSWER1: Fentanyl is under discussion to be added to the HHS 5 panel. The process for changing the federal panel is a very long one and involves approval from a variety of federal offices. This is both good and bad news. This excruciating process has produced a program that has withstood legal challenges, including Supreme Court challenges for 30 years. It is the gold standard testing program, but because of the rock-solid nature of the program, it doesn’t change quickly or easily.
ANSWER2: HHS has recently proposed to establish a new process for publishing and revising the drug testing panels for urine and oral fluid testing. HHS is proposing that they be allowed to change the drug testing panel as necessary to keep up with current drug use trends. The time required to revise the Guidelines through the Federal review process has impeded the HHS’s ability to respond to changes in drug abuse trends, as it can take several years to do so. The HHS proposes to publish the drug testing panel in the Federal Register on at least an annual basis, including any revision to the panel, without the need to undergo notice and comment. Changes to analytes and cutoffs will be based on a thorough review of relevant information, including the current state of the science, laboratory capabilities, cost associated with the change, and benefits of the change to Federal agencies. The notice would include effective dates of the drug testing panel.
ADDRESSING VERIFIED OPIOID PRESCRIPTION AND WORKPLACE SAFETY RISK
QUESTION: Can you describe your process for determining whether a verified opioid Rx poses a safety risk that needs to be reported? It would be helpful to have examples of situations in which a donor is taking an opioid Rx that you have and have not determined to be a safety risk.
ANSWER1: The DOT has clearly stated that the ability to make decisions about safety risk is up to the MRO and that’s why only doctors can be MROs. The most important thing to consider in deciding whether to issue a safety concern is how you will defend that decision if you are asked when under oath in a court of law. Then you must be able to show that your process involves defensible medical judgement and is applied consistently among all of your reviewed cases. There isn’t a formula that will work in every case for every MRO. Remember that drug test results are historical records and the exact time of the dosing and the amount of medication taken is not verifiable, nor is the donor’s plan to use that medication in the future. Be conservative and safety minded in your decision making.
ANSWER2: From an MRO practice perspective, the DOT has an expectation that the MRO discuss prescribed opioid/opiate medications with the donor’s prescribing health care practitioner (HCP) before deciding about a “safety-concern” statement to the employer. All employees subject to DOT testing are considered safety-sensitive, so it is a matter of the MRO determining if there is a significant safety risk if the employee uses the medication as it is prescribed. Factors to discuss with the prescribing physician may include: is the employee currently authorized to use the medication prescribed? Is the prescribing HCP aware of the position/work tasks the employee performs? Is the prescribing HCP comfortable that, if used as prescribed, the drug does not present a risk to the employee safely performing his duties/tasks? Has the HCP considered an alternative medication to the opiate/opioid Rx? Ultimately, it’s your call as the MRO about the safety-concern statement.
ANSWER3: The question as posed, does not differentiate whether the drug test was a federally-regulated (DOT for example) or non-federal (state-based employer for example) test. When performing the MRO interview under the federal system, the MRO is to explain to the donor that any medical information that is learned from the interview may be disclosed if it is determined to pose a potential safety risk. Some refer to this as the “MRO Miranda Warning” although I do not particularly like that phrase, as Miranda rights are derived within a completely different context.
When learning of an opioid prescription, my method of interview is to try to determine the condition for which the medication is being prescribed. If this is learned, I then have some frame of reference as the MRO to understanding if the condition is acute, subacute, or chronic.
Some MROs would say that in any federal situation, one should report a potential safety-sensitive concern. The argument here is that most of these tests are DOT agency tests, and thus, all are being done from a safety-sensitive job perspective. This would be true. However, some would point out that in an acute situation (for example a fractured finger that was seen in the urgent care clinic two weeks ago with a short course prescription that is verified with only ten pills dispensed and the person no longer is taking the medication) there is little likelihood that a safety-sensitive concern still exists. I will admit that my “trigger level” on this is set low; I have a tendency to report safety-sensitive concerns in almost all subacute and chronic situations because it is impossible to determine the level of potential impairment over the telephone.
In the non-federal testing situation, some MROs simply carry their federally-regulated practices along for consistency sake. But there are many non-federal tests that are conducted on employees who do not have safety-sensitive jobs. My suggestion in these cases is to establish a good understanding and relationship with the employer’s HR department or DER representative. Often, I suggest that somewhere within the company drug testing policy, there should be some reference to what jobs within the company are considered safety-sensitive and which ones are not. Having this information spelled out in the policy will save both the MRO and the HR department or DER representative much time and angst over these issues.
As is the case with many MRO functions, practices among MROs on reporting of safety-sensitive concerns vary widely, and there is no “gold standard”.”
Note: These Q&A were compiled by Kent W. Peterson, MD, section editor
FMCSA Notification for Collection Sites and CDL Employers
On September 9, 2022, the Federal Motor Carrier Safety Administration (FMCSA) sent out the notification below. Any questions related to the notification should be directed to FMCSA’s drug and alcohol program office at FMCSADrugandAlcohol@dot.gov
A Notice for Federal Drug Testing Collection Sites & CDL Employers Regarding FMCSA Regulated Employees The US Department of Transportation (DOT) has regulations governing drug and alcohol testing for certain transportation industry employees. These regulations help ensure that the traveling American public can feel safe in their day to day journeys. Part of the effective execution of these regulations relies upon drug testing collection sites. For Federal drug testing programs to operate efficiently and effectively, collection sites play an integral role in making sure the right individuals are administered the right tests.
There are several modes under DOT that have regulations about how employees in their specific part of the transportation industry should be tested. For the Federal Motor Carrier Safety Administration (FMCSA), one of the modes under DOT, only commercial driver’s license (CDL) holders, commercial learner’s permit (CLP) holders, or drivers that should have either a CDL or CLP should be given a DOT drug test with FMCSA specified as the DOT Agency on the custody and control form (CCF). Administering Federal drug tests to anyone other than these groups under FMCSA regulations creates an unnecessary administrative burdens on everyone in the Federal drug testing arena including, employers, drivers, medical review officers, third party administrators, and Federal staff. It is for this reason that FMCSA put together the, “Collection Site Notice” linked below. This notice provides important information for both collection sites and employers to use when determining who should be given what type of test.
Employers: Please keep this notice handy and make sure that anyone involved in drug and alcohol testing at your company has a copy of it. Collection Sites: Please review the attached notice with your staff. Also, we encourage posting the second page of the notice in your collection site, particularly in places where collections are actively taking place.
DOT and FMCSA drug and alcohol testing regulations make it safer for everyone in the United States to get around. This notice will help ensure that these regulations are implemented properly.
Collection Site Notice
FMCSA Drug and Alcohol Testing Program Website
FMCSADrugandAlcohol@dot.gov
Government Resource Websites
ODAPC - DOT
- Office of Drug & Alcohol Policy & Compliance (ODAPC)
- DOT 49 CFR Part 40 Procedures for Transportation Workplace Drug and Alcohol Testing Programs
- Subscribe to the ODAPC Updates and News
- 2022 DOT Random Testing Rates
SAMHSA - HHS
- Medical Review Officer Guidance Manual for Federal Workplace Drug Testing Programs
- Mandatory Guidelines for Federal Workplace Drug Testing Programs using Urine (UrMG)
- Mandatory Guidelines for Federal Workplace Drug Testing Programs using Oral Fluid (OFMG)
NRC
CUSTODY AND CONTROL FORMS (CCFs)
- 2020 Federal CCF for Urine and Oral Fluid Specimens
- 2020 Guidance for Using the Federal CCF for Urine Specimens
- 2017 Federal CCF for Urine Specimens - accepted for urine specimens until August 31, 2023



Medical Review Officer
Certification Council (MROCC)
3231 S Halsted St, Ste Front #167
Chicago, IL 60608
Tel: 847.631.0599
Email: mrocc@mrocc.org
Editor: James Ferguson, DO
Managing Editor: Kristine Pasciak
Copyright 2022 Medical Review Officer Certification Council
ISSN: 2833-0870
MRO Quarterly is an educational publication intended to provide information and opinion to health professionals. The statements and opinions contained in this document are solely those of the individual authors/contributors and not MROCC. MROCC and its editorial staff disclaim responsibility for any injury to persons or property resulting from any ideas or products referred to in this newsletter.
To unsubscribe from MROCC emails, please send an email to mrocc@mrocc.org with the subject unsubscribe.
