DOT ISSUES FINAL RULE AUTHORIZING ORAL FLUID DRUG TESTING
WFQA, LLC Regulatory Compliance Officer
On May 2, 2023, the Department of Transportation (DOT) published a final rule that authorizes employers to use Oral Fluid (OF) drug testing as an alternative testing methodology to urine drug testing in DOT-mandated testing programs. In addition to adding OF testing procedures to 49 CFR Part 40, the revisions also:
- harmonize with the HHS Oral Fluid Mandatory Guidelines (OFMG) which were effective Jan 1, 2020 for federal employee testing programs.
- clarifiy certain Part 40 provisions related to urine drug testing procedures.
- remove text and definitions that are no longer necessary.
- add new definitions, clarifying language, weblinks, etc.
- approve "virtual" Substance Abuse Professional (SAP) evaluations/assessments.
The final rule became effective June 1, 2023, however DOT states that OF testing cannot be conducted by employers until at least 2 drug testing laboratories are HHS certified (approved) for OF drug testing. No laboratories are currently certified. HHS certification of 2 or more labs is not expected before 4th QTR 2023. FAA, FMCSA, FRA, & FTA also issued, with a common preamble, revisions to their drug/alcohol testing regulations (14 CFR Part 120, 49 CFR Parts 382, 219, & 655) to conform with the Part 40 final rule. Of note, is that the FRA will not permit OF drug testing as part of its FRA-mandated post-accident testing program. PHMSA and USCG have determined they do not need to make any changes to their drug testing regulations in order to permit OF testing when it can be conducted.
For the first time in over 30 years when the first DOT drug testing regulations were issued, employers can choose to do something other than urine drug testing. Employers can choose to use OF drug testing for all their required drug testing, in combination with urine drug testing, or not at all, (except for required direct observation collections on non-binary/transgender employees). OF drug testing can be used for all reasons for testing (e.g., pre-employment, random, post-accident, etc.); for any drug test when direct observation urine collection is mandated; as a second specimen collection when a urine specimen is "suspect" or for insufficient quantity (shy bladder ). The employer, not the employee, chooses the specimen for the test reason (e.g., randoms, pre-employment, etc.); and for specific circumstances (e.g. shy bladder, 2nd specimen after suspect specimen is provided, re-collections following a cancelled test). The employer cannot conduct both an OF and a urine drug test on the employee at the start of a DOT test.
The DOT final rule includes OF specimen collection procedures, laboratory analysis processes that mirror the HHS OFMG, and MRO test result verification and reporting procedures that are parallel to urine drug testing. Collectors must undergo qualification training, including mock collections just as urine specimen collectors must do. The current federal custody and control form, approved in 2020 can be used for either OF or urine drug tests. SAMHSA certified laboratories must conduct both screening and confirmatory testing of OF specimens in accordance with the HHS OFMG. The OF testing panel of analytes (drugs) is the same as used in the urine testing programs with the exception that the target analyte for marijuana use is THC, not THC-A as it is in urine testing. There is no required specimen validity testing for OF specimens. Upon the request of the employee/applicant, reconfirmation testing of verified positive OF tests is conducted on the split specimen at a second HHS certified OF drug testing laboratory. Currently certified MROs are not required to complete additional training or certification in order to review and report OF test results.
A few distinguishing features of the OF specimen collection procedures have a significant impact on the implementation of OF testing under DOT rules.
- OF collection device requirements:
- The device must meet FDA, HHS, and DOT specifications.
- The device is specific to the immunoassay screening method used by the laboratory that will test the specimen.
- Devices must have expiration dates (the use of expired device is a fatal flaw).
- The device must be able to collect a specimen that is subdivided into primary and split.
- The device must have an adequate volume indicator to insure that at least 2 mLs of OF are collected.
- The device can collect neat OF specimen or use a buffer solution.
- There is a 10 minute wait period under the supervision of the collector before the OF collection can begin.
- Employee inserts OF collection device into his/her mouth in the presence of the collector.
- Dry mouth procedures are similar to shy bladder protocol, except allowed time is 1 hr and fluid intake is limited to 8 oz of water to provide an adequate OF specimen.
- The collected OF specimen is placed into vials A & B, sealed, initialed, and sent to laboratory with copy of CCF.
Currently there are no OF collection devices that have FDA reagent clearance, meet HHS requirements, and conform to DOT requirements to subdivide the OF collected on a single device. A few devices are reportedly very close to meeting all specifications, however there will be significant lag time for approval, production, distribution and training. Likewise, the collection devices are a key component to the laboratory certification process. The laboratory, in its application to the NLCP must specify the collection device it will use for extracting the OF for analysis and the reagents used for the screening analysis. A laboratory will have to undergo NLCP proficiency testing with the collection device and reagents it intends to use for OF testing and have onsite inspections of the standard operating procedures for screening and confirmation testing. The NLCP estimated time frame for laboratory certification is 3-6 months from the application and submission of required documentation.
Other revisions to Part 40 that became effective June 1, 2023, include:
- DER name and phone number must be on the federal CCF in Step 1A. This information can be preprinted on the CCF or entered by the collector at the start of the drug test process.
- The CDL license number must be used for every FMCSA drug/alcohol test on the CCF/ATF.
- SSN, employer issued ID number, state or other governmental issued ID number (driver's license number) is used for all other DOT agency (i.e. FTA, FAA, FRA etc.) drug/alcohol tests.
- MRO can now "Uncancel" drug test results that were canceled due to an uncorrected flaw.
- Example: A test result that was canceled because copy 2 was unavailable. If the paperwork is obtained within 60 days of the canceled test, the MRO does not need DOT permission to uncancel the result.
- MRO staff members can validate prescription medication information.
- Example: MRO staff can contact the pharmacy to confirm that a donor has a legitimate prescription dispensed at that pharmacy.
- Clarification in the MRO reporting of "Safety Concerns"
- Safety Concerns cannot be recorded on the MRO copy of the CCF. They must be on a separate report or notice.
- The MRO report of Safety Concerns must state the specific nature of the MRO concern.
- Example: "Medications may have an adverse effect on the performance of safety-sensitive duties.
- DOT has approved the use of "virtual" SAP assessments and evaluations, no longer requiring face-to-face, in-person interviews.
- This had been permitted under special COVID provisions and is now a permanent change.
- It is the SAP who decides whether to offer the assessment service in-person or virtually.
- Part 40 rule includes specifications for the virtual interview.
This DOT final rule is certainly a milestone in DOT mandated testing in the transportation industry. The DOT reviewed and considered over 400 public comments submitted on the NPRM published Feb 28, 2022. The final rule, including an extensive, comprehensive preamble, section by section analysis of the revisions to Part 40, and the actual text of amended paragraphs of the rule, is 57 pages in the Federal Register (Vol. 88, No. 84 / Tuesday, May 2, 2023). The FR document can be accessed at: https://www.transportation.gov/odapc/frpubs.
Changing Non-Regulated Urine Sample pH and Creatinine Acceptable Ranges
The acceptance criteria for federal Specimen Validity Testing (SVT) testing was established nearly 30 years ago without appreciable data to support their decisions. An analysis for pH acceptance of urine samples shipped overnight or during times with increased temperatures was not evaluated. The same was true for the selection of urine creatinine normal levels based on nutritional status, sex, physical activity, or weight. Decisions were made with the available data in the scientific literature without "real life" analysis of random urine samples.
SVT has undergone several changes over the past several decades based upon years of testing under strict guidance for federal samples. In past years, the criteria for general oxidants, invalid pH ranges, and creatinine have been adjusted for acceptability. Changes have been made to the drugs in the federal panel (e.g., addition of Hydros, Oxys, 6-AM) as well as changing cutoffs (e.g., cocaine, amphetamines, and others) and even the removal of MDEA to stay current with drug abuse detection. The non-regulated program adopted the SVT values from the federal program. It is time to adopt new pH and creatinine standards for the non-regulated workplace program. Clinical Reference Laboratory (CRL) is offering a new range for elevated pH and dilution criteria for clients which leads to improved turnaround time and fewer invalid or dilute specimens.
pH Criteria
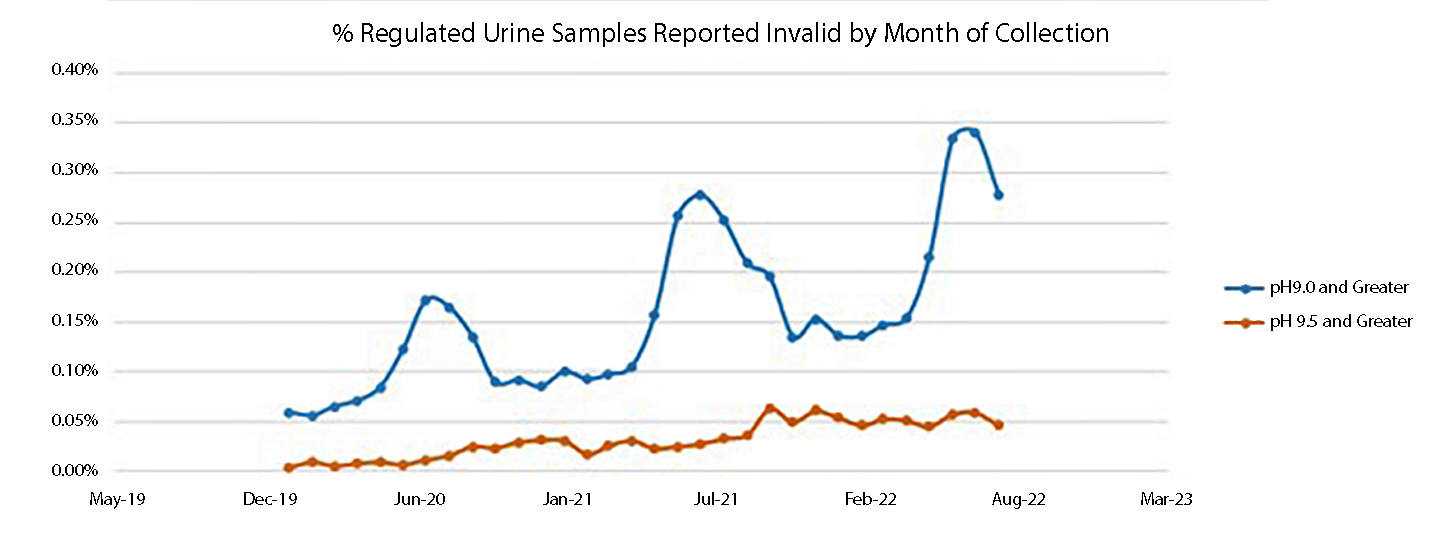
Summer heat causes the pH of urine samples to rise due to bacteria in the urine producing ammonia from byproducts excreted into the urine. This causes the pH of urine to rise above 9 (Invalid Category for DOT) simply because of summer heat and without any action by the donor to "beat the test". The problem of elevated pH is compounded by transportation delays or specimens collected after shipment hours further delaying delivery to the laboratory. An Invalid result is considered a Non-Negative Test and requires a recollection of the donor even though the donor provided the sample properly and laboratory analysis failed to identify a drug positive. This same sample sent in January would most likely be acceptable due to a colder environment during shipment.
Analyzing a week of data at CRL, changing the pH upper range to 9.5 pH revealed that 70% of the "Invalid" samples would be reported as acceptable. However, employers were required to recollect 126 donors samples as their pH was above 9. This means that the donor was not offered a job or a position was not filled. These individuals had negative drug tests which would have made them eligible for hire.
Using CRL data in the graph below, the seasonal variations due to temperature are easily identifiable for the summer months. By using the same set of data and using a pH of 9.5 as the start of the invalid category, the seasonal variations are eliminated. Using existing criteria, the Invalid pH samples are nearly 6-fold higher during summer months. The increase in Invalid results has always existed and but steadily increased each year as the summers are warmer and transportation delays are more common.
Creatinine
The federal program established creatinine range of 20 mg/dL and greater to be considered within the normal range. All samples with a creatinine of less than 20 mg/dL require a specific gravity test to determine if the sample meets criteria as dilute (‹1.003). However both creatinine and specific gravity must meet criteria for dilute which means hundreds of additional tests are performed daily which delay result reporting with little benefit to the employer. For the same pH week comparison, by changing the dilute criteria to 10 mg/dL, 2318 specific gravity tests (82.4%) would be eliminated. It is quite remarkable that the percentage does not change by year or season.
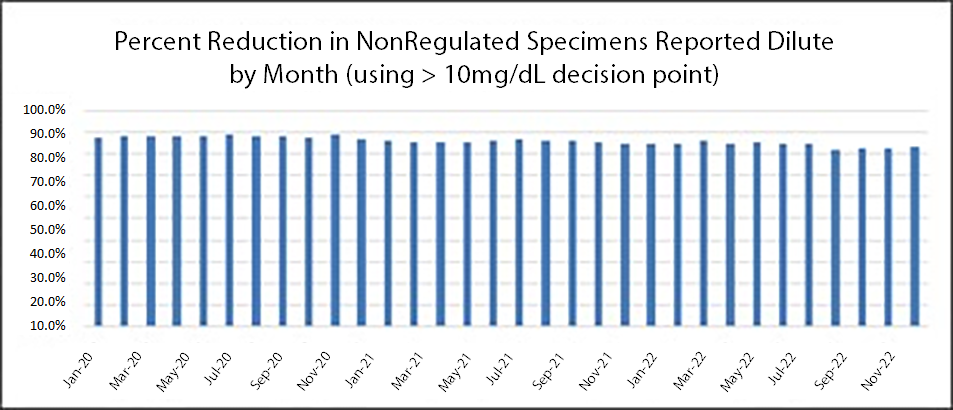
These additional tests are unnecessary. The reporting of a "negative dilute" result does not typically affect an employer's hiring decision. The additional testing results in delayed reporting which is significant for all employment decisions. Medical science continues to promote more fluid consumption to improve overall health. The glass or bottle of water is a standard on the desk by almost everyone.
Summary
Implementation of these two changes can improve the hiring process by reducing delays in reporting without negative impacts to the quality of the test. Non-regulated employers already deviate from the HHS/DOT program by testing different drugs with varying cutoffs. There is no requirement that the HHS/DOT SVT limits be applied to non-regulated testing. By changing these limits, testing delays will be reduced allowing employers to hire and fill job opening more quickly. Donors should not be "punished" for a drug test collected in the summer resulting in an elevated pH due to temperature or delivery time to the laboratory or for drinking larger amounts of fluid as recommended by their physician. Similarly, a negative dilute sample does not impact most hiring decisions. Therefore, by limiting the affected samples, clients are able to extend job offers, fill critical positions and get employees back to work more quickly.
Changes to pH and creatinine may not be applicable for all testing industries. Before changes are made to implement new cut-offs to employers, please recommend that their drug testing policies be reviewed for restrictions.
Consolidated Appropriations Act
DEA Registered-Practitioners
A new one-time, eight-hour training requirement on the treatment/management of patients with opioid or other substance use disorders for all Drug Enforcement Administration (DEA) registered practitioners was enacted on December 9, 2022. This new requirement applies to all DEA-registered practitioners except those that are solely veterinarians. All DEA registrations submitted on June 27, 2023 or later will require practitioners to attest to completion of the new training. This is true for both initial registrations and renewals.
The required training can occur through classroom situations, seminars at professional society meetings, virtual platforms, or through other accredited CME sources. It does not have to be completed in one session. Multiple sessions of relevant training can be combined to fulfill the eight-hour requirement. Additionally, training completed to meet the requirements of the DATA-2000 waiver can count towards the 8-hour training requirement.
Practitioners can fulfill the new training requirement in any of the following ways:
- Holding Board certification in addiction medicine or addiction psychiatry through the American Board of Addiction Medicine (ABAM) or a member board of the American Board of Medical Specialties (ABMS) or the American Osteopathic Association (AOA).
- Graduating in good standing from an accredited medical (allopathic or osteopathic), dental, physician assistant, or advanced practice nursing school in the United States during the 5-year period immediately preceding June 27, 2023 as long as the program included a comprehensive curriculum with not less than 8 hours of training on:
- Treating and managing patients with opioid or other substance use disorders, including the appropriate clinical use of all drugs approved by the Food and Drug Administration for the treatment of a substance use disorder; or
- Safe pharmacological management of dental pain and screening, brief intervention, and referral for appropriate treatment of patients with or at risk of developing opioid and other substance use disorders.
- Completion of not less than eight hours of training on treatment and management of patients with opioid or other substance use disorders from the groups listed below.
- American Society of Addiction Medicine (ASAM)
- American Academy of Addiction Psychiatry (AAAP)
- American Medical Association (AMA)
- American Osteopathic Association (AOA)
- American Dental Association (ADA)
- American Association of Oral and Maxillofacial Surgeons (AAOMS)
- American Psychiatric Association (APA)
- American Association of Nurse Practitioners (AANP)
- American Academy of Physician Associates (AAPA)
- American Nurses Credentialing Center (ANCC)
- Any other organization accredited by the ACCME or the Commission for Continuing Education Provider Recognition (CCEPR)
- Any organization accredited by a state medical society accreditor that is recognized by the ACCME or the CCEPR;
- Any organization accredited by the AOA to provide continuing medical education; or
- Any organization approved by the Assistant Secretary for Mental Health and Substance Use, the ACCME, or the CCEPR.
Additional information can be found on the SAMHSA and DEA websites:
Recommendations for Curricular Elements in Substance Use Disorders Training | SAMHSA
(DEA-DC-067)(EO-DEA260)_Updated_Registration_Certificate_(Final).pdf (usdoj.gov)
(DEA-DC-066)(EO-DEA263)_OUD_Patient_Limits_QA_(Final).pdf (usdoj.gov)
Nuclear Regulatory Commission (NRC) Issues Revisions to 10 CFR Part 26 Fitness for Duty (FFD) Drug Testing Requirements
WFQA, LLC Regulatory Compliance Officer
Just over three years after issuing a Notice of Proposed Rulemaking (NPRM) to amend the NRC Fitness for Duty Drug Testing Requirements (10 CFR Part 26), the final revisions were published November 22, 2022. NRC facilities have until November 22, 2023 to comply with the new requirements for their drug testing programs. The purpose of the Part 26 revisions is to align the NRC's drug testing requirements more closely with the updates made to the U.S. Department of Health and Human Services' Mandatory Guidelines for Federal Workplace Drug Testing Programs (HHS MG) in 2008 and as revised in 2017, and to incorporate lessons learned from the implementation of the NRC's current FFD regulations.
The major provisions of this final rule are:
- Add initial and confirmatory drug testing for methylenedioxymethamphetamine (MDMA) and methylenedioxyamphetamine (MDA).
- Add initial and confirmatory drug testing for four opioid drugs (hydrocodone, hydromorphone, oxycodone, and oxymorphone).
- Add initial drug testing for 6-acetylmorphine (6-AM), a metabolite of heroin, and update the confirmatory drug testing method for 6-AM.
- Lower the initial and confirmatory drug testing cutoff levels for amphetamine, cocaine metabolite, and methamphetamine to conform to the current HHS MG.
- Enhance the detection of subversion attempts by strengthening the testing methods used to identify drugs and drug metabolites in urine specimens with dilute validity test results and in specimens collected under direct observation.
- Permit the collection and drug testing of an oral fluid specimen as an alternative to the collection and testing of a directly observed urine specimen.
- Require MROs to evaluate the elapsed time from specimen collection to testing and exposure to high temperature, as possible causes of some invalid test results due to high pH values (i.e., 9.0—9.5)
- Improve the clarity, consistency, and organization of 10 CFR part 26 by adding and updating definitions; increase flexibility by permitting additional personnel to monitor a donor that is hydrating during a shy-bladder situation; and enhance donor protections by providing additional instruction to same-gender observers used in observed collections and afford due process by requiring MROs to document the date and time that an oral request is received from a donor to initiate the reconfirmation of a specimen.
While these revisions to Part 26 bring the NRC drug testing programs much closer to the HHS MG used for federal workplace testing, there are sill significant differences from DOT and HHS-mandated testing. A few of these differences are highlighted below:
- NRC continues to permit NRC licensees and operators to have drug testing facilities onsite to conduct initial testing for drugs and specimen validity measurements (e.g. creatinine, pH, oxidants, etc.) These instrumented initial testing facilities must use cut-off levels and procedures equivalent to HHS laboratory screening (initial) test requirements. Any specimen positive for drugs or of questionable validity must be sent to an HHS laboratory for confirmation.
- Split specimen collection procedures are optional; determined by the NRC licensee or facility.
- Dilute specimens and certain directly observed specimens must undergo special analysis at the laboratory.
Comments on the 2022 Quest Drug Index (QDI)
Quest Diagnostics recently released their drug index for 2022 (1,2). The QDI tracks drug positive rates in urine specimens collected and tested by Quest and provides data for the federally-regulated and the general workforces. I am going to provide some comment on this year's Index, and I would encourage you to review the report in its entirety.
OVERALL DRUG USE
Figure 1 looks at drug use in the combined workforce from 1988 through 2022. These data represent approximately 9 million urine tests annually.
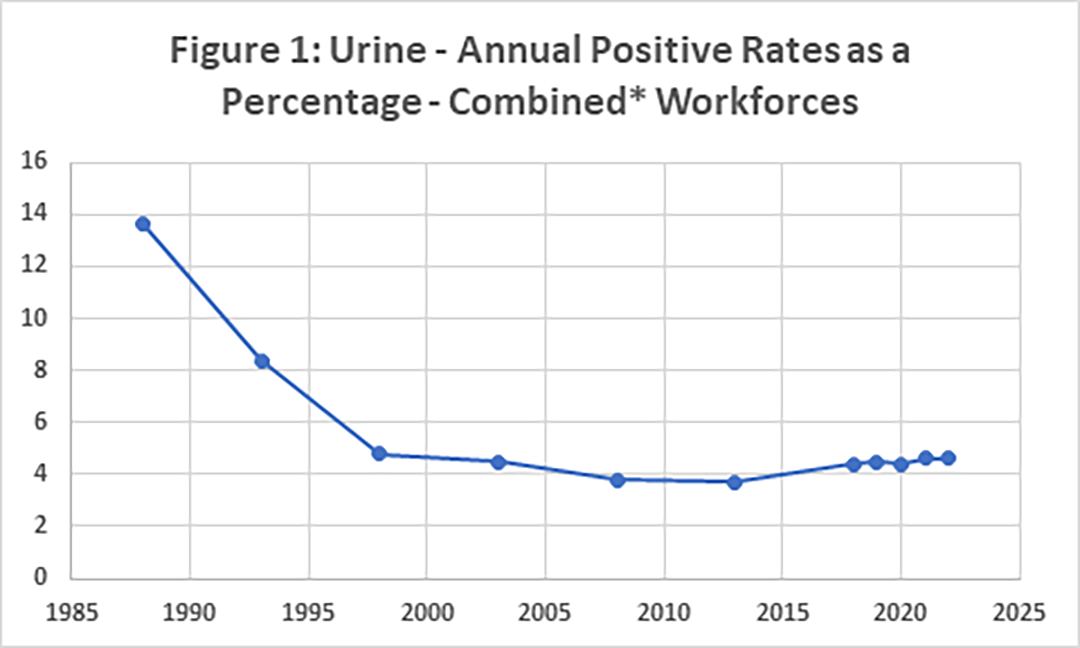
*Combined is the non-regulated and general groups
As you can see this has fallen from 13.6% in 1988 to 4.6% in 2022. The lowest rate was in 2010 to 2012 and that was 3.5%. For the years 2018 to 2022 the federally-regulated workforce were 2.7% (in 2018) to 2.4% (in 2022) and the corresponding percentages in the general workforce were 5.1% to 5.3%. it should be noted that the percentage in the combined workforce in 2021 and 2022 (4.6%) was the highest for a decade.
However, the percentages for post-accident testing were higher as shown in Table 1. These data represent approximately 3 million regulated employees and over 6 million tests in the general workforce.
| Table 1: Positive Rates as a Percentage in Post-Accident Urine Testing | |||||
| 2018 | 2019 | 2020 | 2021 | 2022 | |
| Federal | 4.7% | 4.5% | 4.3% | 4.4% | 4.5% |
| General | 8.4% | 9.1% | 9.5% | 9.7% | 10.3% |
Although the pandemic and lockdown may have had an impact of these numbers, there is also a probable impact form the legalization of marijuana in several states. Today, 21 states have legalized marijuana and 38 states (plus DC) have legalized its medical use. It should be emphasized that under federal law it is still illegal.
More details of marijuana positive rates are included in the QDI (1,2).
Another drug group that is of interest is the amphetamines (amphetamine/methamphetamine) and tabulated below are the percentage positive rates (Table 2).
| Table 2: Positive Rates for the Amphetamines in Urine Testing | |||||
| 2018 | 2019 | 2020 | 2021 | 2022 | |
| Federal | 0.68% | 0.68% | 0.64% | 0.69% | 0.75% |
| General | 1.2% | 1.3% | 1.3% | 1.3% | 1.5% |
The percent increase in this category was 10.3% for federal testing and 25% for general.
The QDI also includes data on the other drug/drug categories tested for and I would encourage you to review that data.
As a point of information, the non-negative rates for specimen validity testing in 2022 were:
- Federal - Acid-Base 0.008%, Oxidizing Adulterants 0%, Substitution 0.017% and Invalid 0.30%
- General - Acid-Base 0.009%, Oxidizing Adulterants 0%, Substitution 0.015% and Invalid 0.31%
Finally, I have included Figure 2, which shows the positive rates for urine, oral fluid, and hair from 2018 to 2022. Obviously, this does not include regulated testing as neither oral fluid nor hair were approved.
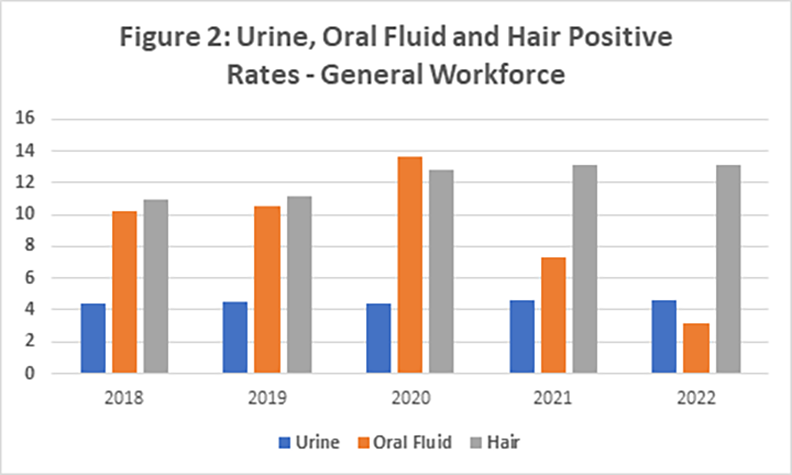
The obvious question is why the drop off in oral fluid testing. The probable answer is that THC testing in oral fluid during 2021 and 2022 decreased significantly and this is the most prevalent drug detected. One could postulate that because of the legalization of marijuana in a large number of states, employers removed it from their pre-employment testing panels.
In summary, the QDI continues to be a valuable resource comparing annual drug use in regulated and general workplaces. In 2022 they documented increases in marijuana use, especially in post-accident testing and in the use of amphetamines.
REFERENCES
- Post-Accident Workforce Drug Positivity for Marijuana Reached 25-Year High in 2022.
https://www.questdiagnostics.com/business-solutions/employers/drug-screening/knowledge-center/drug-testing-index - Quest Drug Index 2022 Tables.
https://www.questdiagnostics.com/business-solutions/employers/drug-screening/knowledge-center/drug-testing-index
ODAPC Back to Basics Series
In May, the Office of Drug and Alcohol Policy and Compliance (ODAPC) announced a short series of reminders called "Back to Basics" for service agents (e.g., collectors, Medical Review Officers, etc). Doing it right is important for protecting the safety of the traveling public, protecting the integrity of the testing process, and making sure that the process is fair to employees. Back to Basics points out the more common issues we hear about regarding the drug and alcohol testing process. This is not a checklist for service agents but a reminder that their role is very important and crucial to the drug and alcohol testing process. We want to remind service agents to "Do It Right the First Time, and Every Time". The second service agent highlighted in the series is the Medical Review Officer (MRO).
Government Resource Websites
ODAPC - DOT
- Office of Drug & Alcohol Policy & Compliance (ODAPC)
- DOT 49 CFR Part 40 Procedures for Transportation Workplace Drug and Alcohol Testing Programs
- Subscribe to the ODAPC Updates and News
- 2023 DOT Random Testing Rates
SAMHSA - HHS
- Medical Review Officer Guidance Manual for Federal Workplace Drug Testing Programs
- Mandatory Guidelines for Federal Workplace Drug Testing Programs using Urine (UrMG)
- Mandatory Guidelines for Federal Workplace Drug Testing Programs using Oral Fluid (OFMG)
NRC
CUSTODY AND CONTROL FORMS (CCFs)
- 2020 Federal CCF for Urine and Oral Fluid Specimens
- 2020 Guidance for Using the Federal CCF for Urine Specimens
- 2017 Federal CCF for Urine Specimens - accepted for urine specimens until August 31, 2023



Medical Review Officer
Certification Council (MROCC)
3231 S Halsted St, Ste Front ###167
Chicago, IL 60608
Tel: 847.631.0599
Email: mrocc@mrocc.org
Editor: James Ferguson, DO
Managing Editor: Kristine Pasciak
©2023 Medical Review Officer Certification Council
ISSN: 2833-0870
MRO Quarterly is an educational publication intended to provide information and opinion to health professionals. The statements and opinions contained in this document are solely those of the individual authors/contributors and not MROCC. MROCC and its editorial staff disclaim responsibility for any injury to persons or property resulting from any ideas or products referred to in this newsletter.
To unsubscribe from MROCC emails, please send an email to mrocc@mrocc.org with the subject unsubscribe.
