2024 California and Washington Laws on Marijuana Use and Employment
WFQA, LLC Regulatory Compliance Officer
Workplace drug testing to detect marijuana use has some significant changes in 2024 for employers in California (CA) and Washington (WA). State statutes AB2188 (CA) and SB5123 (WA), effective January 1, 2024, restrict the employment actions (e.g. hiring, firing, disciplinary, etc.) employers can take based on a drug test positive for marijuana metabolites. Basically, employers cannot take any adverse employment actions based on a drug result reported positive for THCA (Tetrahydrocannabinolic acid). THCA is a non-psychoactive metabolite, and is a precursor to THC (tetrahydrocannabinol), the principal psychoactive component of cannabis. Urine and hair testing for marijuana detects THCA; oral fluid testing for marijuana detects THC (Tetrahydrocannabinol). Thus, employers in CA and WA can use oral fluid drug tests results for employment actions because they detect the psychoactive component of cannabis.
In CA the restrictions apply to both pre-employment and post-employment (e.g. random, post-accident, etc.) drug testing. In WA the restrictions apply only to pre-employment or applicant drug testing. There are, however, exemptions for certain occupations. For instance, in CA the construction industry is mostly exempt, which means construction companies can continue to make employment decisions on employees and applicants based on the presence of the non-psychoactive metabolite(s) of marijuana in urine and/or hair specimens. In the WA statute there is a list of exempt occupations (e.g. firefighters, first responders, 911 dispatchers, corrections officers, positions in the airline and aerospace industries, etc.). These new state laws do not apply to employers conducting federally mandated testing under U.S. Department of Transportation or the Nuclear Regulatory Commission regulations.
So, THE BOTTOM LINE...
- Can employers in CA and WA continue to do drug tests that detect marijuana use?
YES. Employers can take adverse employment actions based on a THC positive drug test. THC is the metabolite detected in oral fluid drug testing. Employers are prohibited from taking employment actions based on urine and hair testing which typically report the non-psychoactive metabolite THCA. - Can employers in CA and WA continue to have drug free workplace policies that prohibit employees from on-duty or on-property marijuana use, being under the influence of marijuana at work, or testing positive for the psychoactive metabolite (THC) of marijuana?
YES. Both the CA and the WA statutes affirm the rights or obligations of an employer to maintain a drug- and alcohol-free workplace. - Can employers continue to conduct urine or hair testing that detects THCA, not THC?
YES, BUT. Test results that detect THCA, the non-psychoactive cannabis metabolite cannot be used as a basis for any adverse employment action, including not hiring an applicant, firing an employee, or other disciplinary action. - What is recommended practice for employers in CA and WA?
CA: Use oral fluid drug testing for all employer required testing. If employers continue to use urine or hair for their drug testing programs, they should consider either dropping marijuana from the testing panel, or ensure that no adverse actions are taken based on a marijuana positive (i.e., THCA) test.
WA: Use oral fluid testing for preemployment drug testing that includes marijuana, cocaine, amphetamines, opiates/opioids, and PCP on the testing panel. - Does any drug test positive for THC or THCA establish impairment?
NO. There are no tests that establish cannabis impairment. State laws for "per se" driving under the influence of marijuana are based on THC blood level; however, they vary greatly from state to state.
Fentanyl Trending in Urine and Oral Fluids at CRL
The evolution of fentanyl from an effective narcotic to treat severe pain to a national abuse crisis is a story which has played out daily on television and in the press. Fentanyl is found with a variety of other drugs of abuse such as heroin, cocaine, and methamphetamine but is also found on the street masquerading as Xanax and Oxycontin. The first U.S. border seizure of 23 pounds of fentanyl occurred in California in 2014. That total increased to 23,700 pounds in 2022. In 2023, the DEA reported a seizure of 79 million fake pills which was enough for 50 million deadly doses. Base chemicals to make fentanyl have been easy to obtain and are very easy to manufacture and transport by drug cartels
There have been three recent waves of narcotic/opioid abuse. The first wave started around 2000 with the rise in oxycodone and hydrocodone prescriptions distributed through "fake pain clinics" in strip malls along the east coast of the U.S. This continued for many years until restrictions in opioid production and law enforcement curbed illegal prescribing and diversion. The second wave began around 2010 due to the reduction and restrictions with prescription opioids which effectively caused an increase in heroin use as it was widely available and inexpensive. The U.S. is currently experiencing the third wave with the widespread distribution of fentanyl in street pills, vapes, and injectables. By 2021 there were 100,000 annual overdose deaths of which 70% were attributed to fentanyl. This is because fentanyl is 100-fold stronger than morphine and 50-fold stronger than heroin. Overdose death occurs very quickly without Narcan administration.
CRL has been testing for fentanyl for several years. The initial testing requests were for professional monitoring programs due to diversion of prescription fentanyl and use by medical personnel. However, the fentanyl epidemic has caused employers to add fentanyl to their routine drug testing panel. The U.S. Depart of Health and Human Services (HHS) is considering adding fentanyl or its metabolite norfentanyl to the federal panel and in the process of establishing cutoffs. HHS will need to add fentanyl to both the urine and oral fluid federal panels at the same time which will lengthen the time to market because there is no FDA approved immunoassay screen for oral fluids. It may take years to add fentanyl to a federal panel if immunoassay vendors need to meet the FDA requirements for an immunoassay.
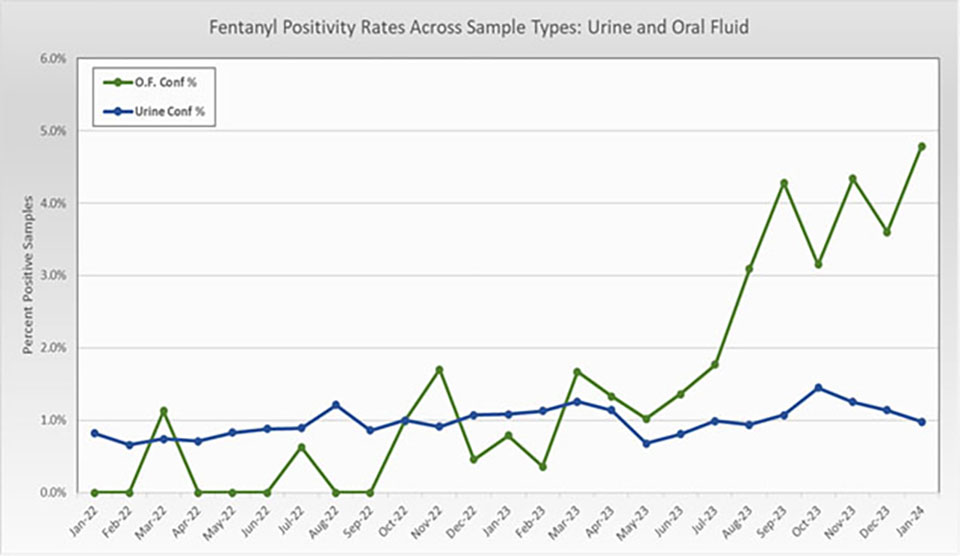
The data presented below is based on workplace drug testing conducted at CRL. The data has been reviewed to eliminate Professional Monitoring Programs, rehabilitation, and probation parole groups. The overall positive rate for urine and oral fluids for January 2022 to January 2024 is displayed in Figure #1. The overall rate for urine is around 1% with oral fluids higher around 3-5%. Oral fluids positivity rates demonstrate more variability due to the lower number of requested tests.
Figure 1: Positivity rates for Urine and Oral Fluids
Urine Data Review:
The common thought is that fentanyl and its metabolite, norfentanyl, are difficult to detect due to low urinary concentrations. However, that is not true. The mean value for urinary fentanyl level is 257 ng/mL, median value of 22 ng/mL and a maximum of 36,199 ng/mL. Norfentanyl values are approximately 4-fold higher than fentanyl. CRL data clearly indicates that fentanyl is being abused and detection is possible with a cutoff level of 1 ng/mL.
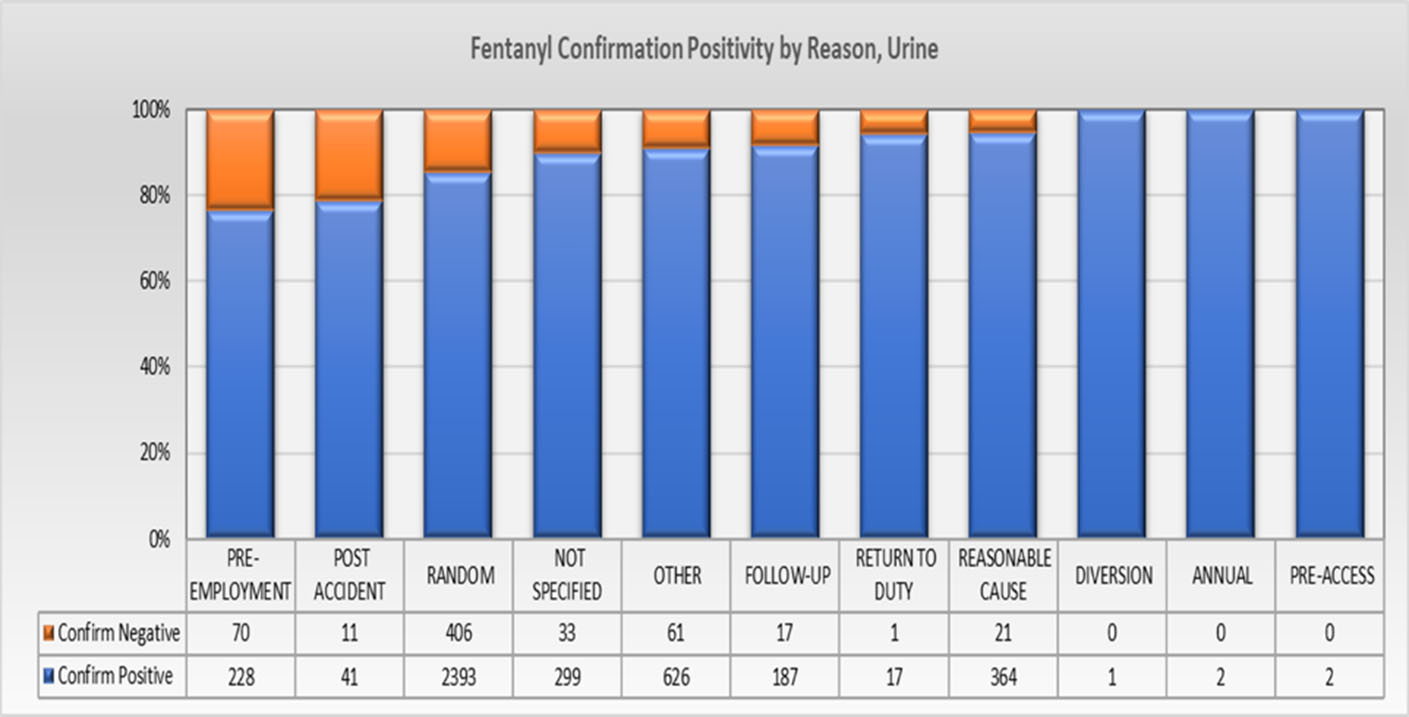
The data was further sorted by Reason for Test in Figure 2. It was very surprising that the highest concentration of fentanyl positive samples occurred in the Random category and not Pre-Employment even though pre-employment samples account for around 50% of the tests. The prevalence of fentanyl in Reasonable Cause tests indicates that employees are using fentanyl and consequently creating unsafe working conditions on the job.
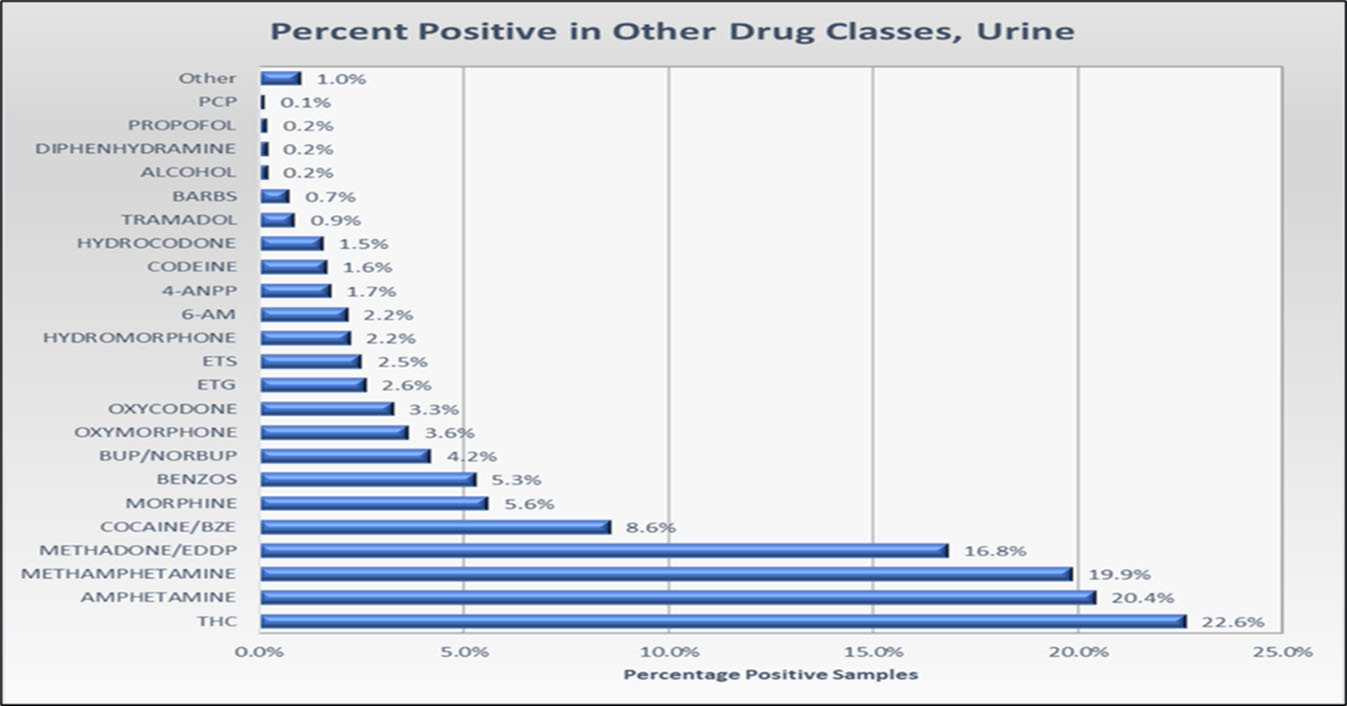
Fentanyl results were also analyzed for positives in combination with other drugs in the testing panel. The most common drug found was marijuana at 22.6% with methamphetamine a close second at around 20% as illustrated in Figure #3. It was also unexpected to find fentanyl in combination with methadone at 16.8% which is nearly double that of cocaine at 8.6%. Other drugs are listed by their percentage found.
Figure #3 Drug Combo with Fentanyl
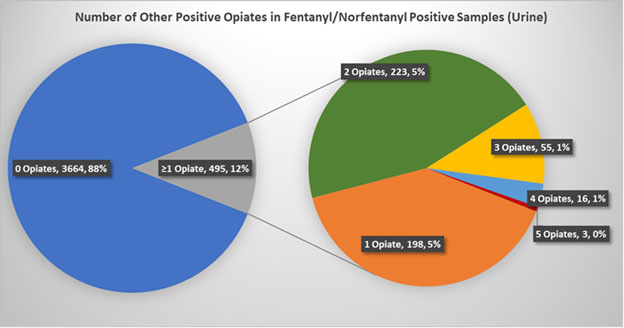
It was thought that opioids and fentanyl would be the most common combination, but in fact 88% of the fentanyl positive samples did not contain an opioid. The opioid category is further divided in Figure #4. When analyzing this category, it is most common to find 1 or 2 opioids but several samples contained up to 5 opioids.
Oral Fluid Review
Testing in oral fluids was done by LC-MS/MS screening and confirmation as an immunoassay reagent is not available. All oral fluid collections were completed using OraSure Intercept collectors. Oral fluids are reported as diluted values because of the Intercept device buffer and results need to be multiplied by 3 to obtain neat values.
The concentrations of fentanyl in oral fluids are substantial. The median value for fentanyl was 19.7 ng/mL or nearly 60 ng/mL as a neat value. Concentrations went to the Limit of Detection of 2000 ng/mL or 6000 ng/mL as neat. Unlike urine, fentanyl is 4-fold higher than norfentanyl in oral fluids. This certainly suggests that different compounds may be promoted as the primary analyte for reporting
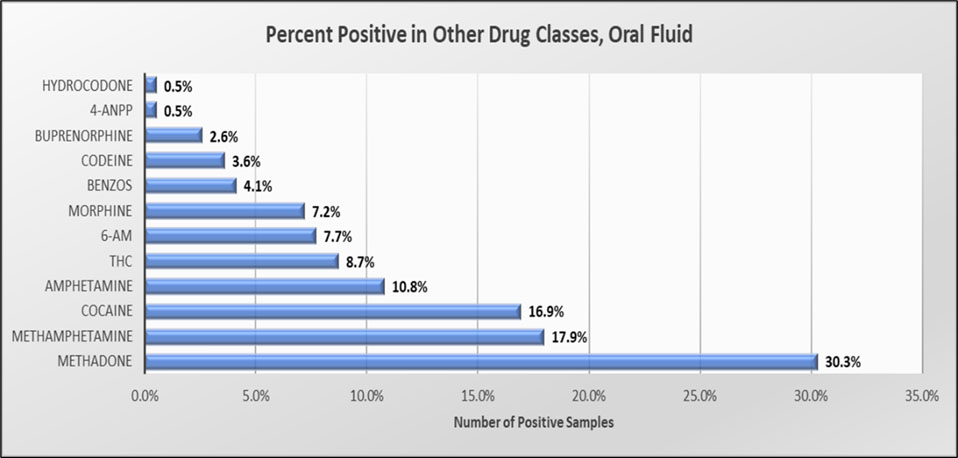
Fentanyl in combination with other drugs was also significantly different in oral fluid (Figure #5) with methadone found in 30% of the samples. Methamphetamine was the second most common poly- positive with marijuana falling to 5th place at 8.7%. It was also noted that 90% of the positive oral fluid samples did not contain any additional opioids.
Figure #5 Combination Drugs in Oral Fluid
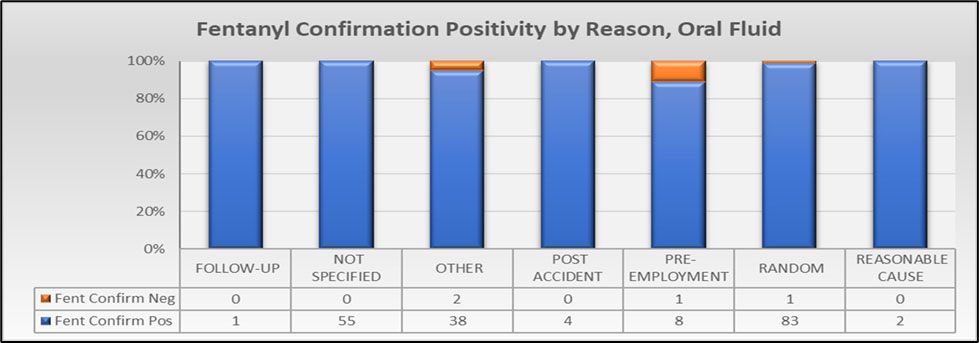
Oral fluid positivity rate by Reason for Test is shown below in Figure #6. Similar to urine, the Random reason for test had the most positive samples with other major categories including Not Specified and Other. These nonspecific categories were marked by the collector during collection and CRL did not pursue a follow up to determine an accurate category. Although pre-employment is a major reason for test, it did not have substantial positive rates.
Figure #6 Fentanyl Confirmation Positivity by Reason for Test
Summary
Testing for fentanyl has become important for employers to maintain a safe work environment. CRL has 1600 testing panels which include fentanyl. The reason for the high number of panels is that employers have very specific panels with various drugs and cutoffs. It does not appear that the distribution of fentanyl slowed and employers must continue to be aware of the prevalence of fentanyl in their workplace for their employee safety.
The federal program announced in the March 5, 2024 Drug Testing Advisory Board (DTAB) meeting that fentanyl screen and confirmation limits are set for 1 ng/mL for urine and oral fluids. It is now up to the immunoassay vendors to create a 1 ng/mL oral fluid screen.
Unfortunately, there are new and even more potent drugs on the horizon that are ready to replace fentanyl. The nitazine family of drugs are 40-800 times more potent than fentanyl and will be difficult to detect as urine detection levels will even be lower. A drug this potent is very difficult to dilute to a reasonable level for distribution in clandestine pills. Fatalities will likely be quite high and will likely limit its long-term distribution.
Update on Medicinal Cannabis Use in Canada
Reprinted with permission from The Drug & Alcohol Testing Association of Canada (DATAC). DATAC Article: Update on Medicinal Cannabis Use in Canada, Jan 3, 2024. Available at: https://datac.ca/update-on-medicinal-cannabis-use-in-canada/.
In December of 2023 Health Canada published updated data on cannabis use for medical purposes. The Canadian government originally granted access to medical cannabis to individuals diagnosed with severe illnesses that could not be managed using conventional therapies in 2001. Subsequently, in 2018, the recreational use of cannabis became legalized in Canada.
Data on medical client registrations
According to the update, medical client registrations with federally licensed sellers decreased by 4% from 213,099 in March 2023 to 203,804 in June 2023.
In addition, the number of individuals registered with Health Canada for personal and designated cultivation of cannabis for their own medical purposes also decreased by 12% from 19,076 in March 2023 to 16,778 in June 2023.
The majority of registrations for personal/designated cannabis production were from Ontario, British Columbia, and Quebec.
Data on daily amounts authorized by health care practitioners
The update shows that the majority of individuals who access cannabis for medical purposes under the Cannabis Act and its regulations obtain quality-controlled cannabis from a federally licensed seller of cannabis for medical purposes. Moreover, the average daily amount of cannabis authorized by health care practitioners for individuals who access cannabis from federally licensed sellers has remained at 2 grams per day since 2018.
It was also found that the average daily amount authorized by health care practitioners for individuals who are registered with Health Canada for personal or designated production was 34 grams per day.
There were 5,394 health care practitioners associated with registrations made in the previous 12 months with federally licensed sellers.
Furthermore, there were 1,089 health care practitioners associated with active personal/designated production registrations, 243 of whom authorized amounts equal to or above 25 grams per day, and 18 practitioners authorized amounts equal to or above 100 grams per day.
Finally, the data shows that the majority of health care practitioners authorizing cannabis amounts equal to or above 25 grams per day were located in British Columbia and Ontario, and all health care practitioners authorizing amounts equal to or above 100 grams per day were located in these two provinces.
MRO Responsibilities Under
10 CFR 26 (NRC) Fitness for Duty Regulation:
Overview and Subversion vs Substitution/Adulteration
Nuclear Regulatory Commission (NRC) regulations regarding Fitness for Duty (FFD) were initially published in the Federal Register, Volume 54, #108, June 7, 1989. The summary on page 24468, states, in part:
"...The Commission is taking this action to significantly increase assurance of public health and safety. The scientific evidence is conclusive that significant decrements in cognitive and physical task performance result from intoxication due to illicit drug abuse, as well as the use and misuse of legal substances..."
NRC regulations include specific requirements for workplace drug testing similar to those found in the Department of Transportation's rule 49 CFR 40. However, 10 CFR 26 is a true, comprehensive FFD program and extends far beyond workplace drug testing. The specific performance objectives for 10 CFR 26 FFD programs are found in 26.23:
§ 26.23 Performance objectives
Fitness-for-duty programs must
- Provide reasonable assurance that individuals are trustworthy and reliable as demonstrated by the avoidance of substance abuse;
- Provide reasonable assurance that individuals are not under the influence of any substance, legal or illegal, or mentally or physically impaired from any cause, which in any way adversely affects their ability to safely and competently perform their duties;
- Provide reasonable measures for the early detection of individuals who are not fit to perform the duties that require them to be subject to the FFD program;
- Provide reasonable assurance that the workplaces subject to this part are free from the presence and effects of illegal drugs and alcohol; and
- Provide reasonable assurance that the effects of fatigue and degraded alertness on individuals' abilities to safely and competently perform their duties are managed commensurate with maintaining public health and safety.
[73 FR 17182 Mar. 31, 2008]
Just as the NRC FFD Program is far more than workplace drug testing, the role of the Medical Review Officer (MRO) is far more than the review and interpretation of laboratory results. Under NRC regulations, the role of the MRO is defined as follows:
§ 26.183 Medical review officer
- Responsibilities. The primary role of the MRO is to review and interpret positive, adulterated, substituted, invalid, and dilute test results obtained through the licensee's or other entity's testing program and to identify any evidence of subversion of the testing process. The MRO is also responsible for identifying any issues associated with collecting and testing specimens, and for advising and assisting FFD program management in planning and overseeing the overall FFD program.
From this definition, one can see that the MRO has a specific role in identifying subversion throughout the entire process and in advising and assisting the FFD program as a whole. The MRO under NRC regulations is deeply imbedded within the company's entire FFD program; he/she is not simply a role player.
Subversion vs Substitution/Adulteration
NRC and DOT regulations are similar in that both define "substituted" and "adulterated" specimens via specific laboratory parameters. Only the laboratory can certify a specimen as "substituted" or "adulterated." There is no MRO judgement on the matter.
However, NRC FFD rules are more comprehensive in this area as well and, as noted above, expect the MRO to play an important role in identifying any evidence of subversion. Subversion is specifically defined:
§ 26.5 Definitions
Subversion and subvert the testing process mean a willful act to avoid being tested or to bring about an inaccurate drug or alcohol test result for oneself or others at any stage of the testing process (including selection and notification of individuals for testing, specimen collection, specimen analysis, and test result reporting), and adulterating, substituting, or otherwise causing a specimen to provide an inaccurate test result.
No other federal workplace drug testing includes the term "subversion" in their regulation. This is unique to NRC FFD Programs. Here, the MRO is expected to exercise clinical judgment in evaluating specimens that may have been an attempt to subvert the testing process. As part of that evaluation, it is often important to contact the laboratory and request additional information that the laboratory obtained but did not include in the final report, i.e. actual creatinine and pH levels. The following case studies demonstrate how this is accomplished.
Subversion Cases: Obvious & Not Quite so Obvious
CASE #1
1st collection 07:47, temp 102.5, validity testing WNL, negative screening
2nd collection 08:27 (after 16 oz water), observed, temp 96.1, GC/MS confirmed THC 10 ng/ml
NOTE:
Confirmation testing cut-off levels do not apply in NRC For-Cause/Reasonable Suspicion testing or when specimens are dilute. Confirmation testing is performed to the LOQ defined by the laboratory. (4/5/2024-The author has amended the NOTE statement above to include "confirmation" and to provide clarification. More on this specific topic will be included in a future newsletter.)
Additional information obtained from the lab:
1st collection Cr 63.9, pH 7.2, nothing even within 50% of immunoassay screening cut-off, totally "clean"
2nd collection Cr 136.5, pH 7.0
DISCUSSION:
Collector noted that the donor did not appear to be ill
2nd collection only 40 min after the 1st collection; most unusual
With hydration, Cr decreases, it does NOT increase
With hydration, pH should slightly increase (lower conc H ion equivalent), NOT decrease
NOTE:
pH scale is a logarithmic scale, every 1 unit = 10 fold change H ion conc
At LOD/LOQ testing, 4.7 ng/ml THC should have been in the 1st specimen when correcting for difference in Cr level. LOQ for THC at the lab was 2 ng/ml.
NOTE:
Under 10 CFR 26, this is a Refusal to Test due to Attempted Subversion
Under 49 CFR 40, this is a verified neg result (THC < 15 ng/ml cut-off
level); results from the 1st collection are disregarded.
CASE #2
1st collection 09:02, temp 88.3, validity testing WNL, negative screening
2nd collection 09:37, observed, temp 93.5, validity testing WNL, GC/MS
confirmed THC 1654 ng/ml
DISCUSSION:
2nd collection only 35 min after the 1st collection; most unusual
Only rarely is a specimen with temp < 90 or > 100 a valid collection
ALWAYS recollect under direct observation
NOTE:
Under 10 CFR 26, this is a Refusal to Test due to Attempted Subversion
Under 49 CFR 40, this is a verified THC pos only, not a refusal to test;
results from the 1st collection are disregarded.
CASE #3
1st collection 07:55, temp 84.4, validity testing WNL, negative screening
2nd collection 10:15, observed (after 32 oz water), temp 93.8, validity testing
WNL, negative screening
Additional information obtained from the lab:
1st collection Cr 262, pH 5.4, nothing even within 50% of screening cut-off, "clean"
2nd collection Cr 46.7, pH 6.5, nothing even within 50% of screening cut-off, "clean"
DISCUSSION:
2nd collection 2 hrs, 20 min and 32 oz water after first collection
2nd collection Cr decreased by a factor of 5.6 compared to 1st collection
2nd collection pH 6.5 indicates H ion conc decreasing by a factor of 12.6 (5981.1 nEq/L to 316.23 nEq/L, from faa/gov Table converting pH to nEq/L, ref below).
If dilution decreased Cr by a factor of 5.6, H ion conc should have done the same, resulting in a pH of 5.8. The huge change in pH in only 2 hrs 20 min between the two specimens is not physiologically possible.
NOTE:
Under 10 CFR 26, the donor does have the opportunity to "prove" that
he/she is capable of physiologically producing the specimens as obtained.
However, "the burden of proof resides solely with the donor, who must provide legitimate medical evidence within 5 business days that he or she produced" these results.
NOTE:
Under 10 CFR 26, this is a Refusal to Test due to Attempted Subversion
Under 49 CFR 40, this is a verified neg result; results from the 1st
collection are disregarded.
These cases demonstrate one of the primary roles of the MRO under 10 CFR 26:
"to identify any evidence of subversion of the testing process." The entire testing process surrounding that donor on that day is to be reviewed and evaluated. No data is disregarded; all data needs to be consistent with normal human physiology unless the donor can provide medical evidence that their physiology differs from the normal.
Substance Abuse Program Administrators Association (SAPAA)
In the complex field of workforce drug and alcohol testing, the Substance Abuse Program Administrators Association (SAPAA) strives to serve as a resource for knowledge, professionalism, and best practices. For nearly 32 years, we've been committed to supporting the industry, though we see our role as one piece of a much larger community effort.
Central to our mission is the professional growth and recognition of individuals and organizations working within our industry. SAPAA aims to offer a supportive platform for learning, collaboration, and the sharing of best practices. Our membership includes third-party administrators, in-house program administrators, Medical Review Officers/Team Members, HHS-certified laboratories, Substance Abuse Professionals, testing device manufacturers, and collection sites/collectors. Together, we work towards enhancing the standards that our industry relies upon.
Our efforts to advocate for fair, practical, and scientifically grounded regulations are a testament to our commitment to improving operational excellence and safeguarding the interests of employers and employees alike. We engage in these discussions and processes with the aim of ensuring that testing procedures are carried out with the highest level of integrity and ethical responsibility.
Perhaps what we take the most pride in is the vibrant community of professionals we've helped foster over the years. This community shares a united goal: to improve the quality and reliability of workforce drug and alcohol testing services. Our annual conferences, workshops, and networking events are not just about us — they are about providing valuable opportunities for our members to connect, exchange ideas, and work together on initiatives that promote innovation and excellence in our field. The sense of community and collective purpose that has emerged is something we deeply cherish and are grateful to be a part of.
We recognize that our journey is a shared one, made possible by the contributions and engagement of every SAPAA member. It is this collective effort that continues to drive us forward, shaping the future of workforce safety and health.
If you'd like to learn more about SAPAA, please visit us at www.sapaa.com.
ODAPC - DOT
- Office of Drug & Alcohol Policy & Compliance (ODAPC)
- DOT 49 CFR Part 40 Procedures for Transportation Workplace Drug and Alcohol Testing Programs
- Subscribe to the ODAPC Updates and News
- 2024 DOT Random Testing Rates
SAMHSA - HHS
- Medical Review Officer Guidance Manual for Federal Workplace Drug Testing Programs
- Mandatory Guidelines for Federal Workplace Drug Testing Programs using Urine (UrMG)
- Mandatory Guidelines for Federal Workplace Drug Testing Programs using Oral Fluid (OFMG)
NRC
CUSTODY AND CONTROL FORMS (CCFs)
- 2020 Federal CCF for Urine and Oral Fluid Specimens
- 2020 Guidance for Using the Federal CCF for Urine Specimens



Medical Review Officer
Certification Council (MROCC)
3231 S Halsted St, Ste Front ###167
Chicago, IL 60608
Tel: 847.631.0599
Email: mrocc@mrocc.org
Editor: James Ferguson, DO
Managing Editor: Kristine Pasciak
©2024 Medical Review Officer Certification Council
ISSN: 2833-0870
MRO Quarterly is an educational publication intended to provide information and opinion to health professionals. The statements and opinions contained in this document are solely those of the individual authors/contributors and not MROCC. MROCC and its editorial staff disclaim responsibility for any injury to persons or property resulting from any ideas or products referred to in this newsletter.
To unsubscribe from MROCC emails, please send an email to mrocc@mrocc.org with the subject unsubscribe.